Esterification of acetic acid with ethanol in carbon dioxide
Lynnette A. Blanchard and Joan F. Brennecke*
Department of Chemical Engineering, University of Notre Dame, Notre Dame, IN 46556, USA.. E-mail: jfb@nd.edu
First published on 26th January 2001
Abstract
Simply applying CO2 pressure on a liquid phase, reversible, equilibrium-limited reaction can enhance its equilibrium conversion. In particular, we show that the equilibrium conversion of the esterification of acetic acid with ethanol can be shifted from 63% in neat solution to 72% in CO2 at 333 K and 58.6 bar.
Green ContextThe ability to push equilibrium reactions further to completion is a key factor which is often achieved by the continuous removal of one component, or the use of large excesses of another. The latter is obviously not well suited to green chemistry, whereas the former may often be technically difficult. This paper explores the ability of a novel solvent, supercritical CO2, to influence the final position of equilibrium. In the esterification of ethanol and acetic acid, it is shown that the final equilibrium position is shifted significantly towards products compared to conventional solvents. Such an approach may help in designing more environmentally benign processes, where equilibrium position is importantDJM |
Introduction
Supercritical carbon dioxide (scCO2) has received much interest in the past several years as an alternative solvent to replace hazardous organic liquids. Most research on reactions with high-pressure CO2 has focused on the solvent’s possible effects on reaction rates, selectivities and yields.1 For example, researchers have obtained higher enantioselectivities for an asymmetric catalytic hydrogenation reaction using scCO2 as the solvent instead of conventional organic solvents.2 CO2 does not have to be in the supercritical state to be a viable reaction media. Recently the indium-mediated allylation of aldehydes was carried out in liquid CO2.3 Numerous industrial processes employing carbon dioxide as either an extraction solvent or as a reaction medium have come online in the last two decades. For instance, DuPont recently announced the construction of a plant to polymerize fluoromonomers in CO2.4This work investigates the effect of using CO2 as a solvent for an equilibrium limited reversible reaction, the esterification of acetic acid with ethanol [eqn. (1)].
| MeCO2H + EtOH ⇌ MeCO2Et + H2O | (1) |
The formation of ethyl acetate and water from the esterification of acetic acid with ethanol is equilibrium limited in the liquid phase for both the neat form and in various non-reactive solvents. Since this reaction progresses very slowly, a strong acid catalyst, typically sulfuric or hydrochloric acid, is generally added to increase the rate of the reaction; however, too much catalyst may cause dehydration of the alcohol or isomerization. In addition, the acids are corrosive and hazardous. It is important to note that the presence of an acid catalyst does not alter the equilibrium constant for the reaction, which is a function of temperature only, nor does it change the extent of conversion—it just enhances the reaction rate.11 The most effective way to increase product formation is to carry out this reaction in such a manner so as to remove one of the products, usually water, during the reaction process, effectively pushing the equilibrium to the right. One option is reactive distillation; however, the components in this reaction form highly non-ideal liquid mixtures, with the possibility of forming several different azeotropes.12,13
The impetus for this work is to explore the effect high-pressure CO2 may have on the uncatalyzed equilibrium-limited esterification reaction. The equilibrium constant is a function of temperature only but even for single-phase reactions the pressure can affect the conversion. The reaction equilibrium constant in the gas phase or a gas swollen liquid phase that can be modeled with an equation of state is given by eqn. (2).
 | (2) |
Another way that the introduction of high-pressure CO2 might affect the conversion is through the formation of multiple phases. If one of the products is preferentially transferred to an additional phase, this can effectively enhance the conversion to products. For example, the formation of an aqueous-rich liquid phase would likely result in higher product yield. As a result, in this study we simultaneously investigate the reaction progression and the phase behavior. At the conditions investigated here, 333 K and 58.6 bar, we would certainly anticipate the formation of at least two phases, a liquid phase and a relatively dense gas phase. There is also the possibility of forming an additional liquid phase since several ternary systems comprised of species from eqn. (1) exhibit liquid–liquid–vapor behavior: Ethanol–ethyl acetate–H2O at 70 °C and atmospheric pressure.14CO2–acetic acid–H2O at 20, 30, 40 °C and 48.5–77.5 bar.15,16CO2–ethanol–H2O at 35, 40 °C and 67.8–103.1 bar.17–19This work measures the phase and reaction equilibria of an equilibrium-limited reaction under CO2 pressure. The use of high-pressure carbon dioxide, an environmentally benign compound, provides a mechanism to shift the extent of reaction or yield. This is accomplished in the absence of corrosive, hazardous acidic catalysts, that can only affect the reaction rate and not the equilibrium conversion.
Results and discussion
The progression of the esterification of acetic acid under CO2 pressure is shown in Fig. 1. The concentrations were normalized to the amount of ethyl acetate due to small differences in sample quantities used. The variable volume view cell was initially loaded with an approximate reactant to product mole ratio of 3 to 1. At 333 K and 58.6 bar the liquid phase present at the beginning of the reaction contained about 30 mol% CO2. The liquid phase composition was used as the basis for the extent of reaction calculation since the concentrations of reactants and products in the vapor phase are relatively small, as described below. The formation of ethyl acetate was relatively rapid in the first 15 days, with full chemical equilibrium of the esterification reaction being reached after 42 days, as indicated by the relatively constant compositions. The reaction is slow because we chose to run the reaction in as simple a form as possible, i.e., without added catalyst. The final reactant to product equilibrium mole ratio was ca. 1∶2.5. This would be 72% conversion to products, if one had started with all reactants. The reaction was monitored via the window of the view cell, through which a vapor and liquid phase of approximately equal volumes were observed throughout the duration of the experiment. Thus we did not observe liquid–liquid–vapor equilibrium at any time during the course of the reaction.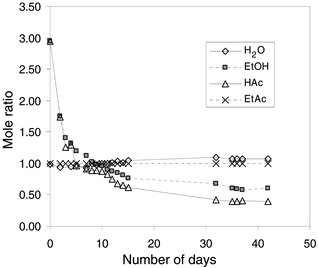 | ||
| Fig. 1 Progression of the reaction of acetic acid (HAc) and ethanol (EtOH) to ethyl acetate (EtAc) and water in CO2 at 333 K and 58.6 K bar. | ||
The composition of the liquid phase as a function of time is shown in Fig. 2. Concentrations of the reactants, acetic acid (HAc) and ethanol (EtOH), decrease as a function of time from 0.23 and 0.23 mole fractions to 0.08 and 0.12, respectively. In response, the product concentrations increase from 0.12 mole fraction for both water and ethyl acetate to 0.27 and 0.25 mole fractions over the same time period. The lines in Figs. 1 and 2 representing the conversion of acetic acid and ethanol should be coincidental owing to the equimolar nature of the esterification reaction. The same is true for the water and ethyl acetate formation lines. However, as seen in Fig. 2, the mole fraction of H2O in the liquid phase is greater than the mole fraction of ethyl acetate throughout the reaction. This may be due to the higher solubility of ethyl acetate in the CO2-rich gas phase, as seen in Fig. 3. The mole fraction of CO2 in the liquid phase decreased slightly from 0.30 to 0.26 over the duration of the reaction. The slight decrease of CO2 solubility in the liquid phase can be attributed to the formation of H2O, a compound with which CO2 exhibits very low mutual solubilities.20
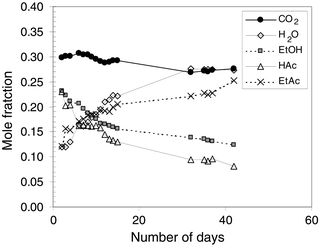 | ||
| Fig. 2 Liquid-phase compositions in the esterification of acetic acid (HAc) with ethanol (EtOH) in CO2 at 333 K and 58.6 bar. | ||
 | ||
| Fig. 3 Vapor-phase composition in the esterification of acetic acid (HAc) with ethanol (EtOH) in CO2 at 333 K and 58.6 bar. | ||
Fig. 3 represents the vapor phase compositions of the esterification reaction. The concentration of CO2 is relatively constant at 0.86 mole fraction and it comprises the majority of the vapor phase. The solubilities of the reactants and products in the vapor phase are somewhat low, the highest concentration being 0.05 mole fraction of ethyl acetate. The amount of acetic acid, ethanol and water in the vapor phase were 0.04, 0.03 and 0.02 mole fraction, respectively. The slight difference in concentrations may account for the previously mentioned discrepancies in the liquid-phase compositions. The solubilities of the two reactants in the CO2-rich gas phase are rather similar. Conversely, the ethyl acetate has a significantly higher solubility in the gas phase than the other product, H2O.
It should be noted that the presence of H2O and CO2 will inevitably result in the production of some carbonic acid. In fact, several hundred bar of CO2 pressure applied to pure water results in the reduction of the aqueous phase pH to as low as 2.8–2.95.21 Since the esterification of acetic acid with ethanol is acid catalyzed this might have an influence on the rate of the reaction. However, as pointed out above, catalysts enhance rates but do not affect the reaction equilibrium constant. Since the reaction was started with a reactant to product mole ratio of 3 to 1, carbonic acid would have been present from the beginning of the reaction. Since the reaction rate is quite slow, as seen from the slow change in compositions in Fig. 1, we conclude that any influence of carbonic acid on the reaction rate is small. Nonetheless, the presence of small amounts of carbonic acid in the liquid phase could have some effect on the activity (or fugacity) coefficients of the various components in the liquid phase, which determines product distribution and, ultimately, the equilibrium conversion observed experimentally.
The conversion achieved using high-pressure CO2 as an environmentally benign solvent is a significant improvement over the neat reaction, which only proceeds to 63% conversion at 60 °C, as indicated in the literature11 and confirmed by measurements in our laboratory. Thus, CO2 can be used to enhance the equilibrium conversion of a reversible, equilibrium-limited reaction. For the esterification of acetic acid with ethanol, the mechanism appears to involve both the preferential solubilization of one of the products, ethyl acetate, in the CO2-rich gas phase, and pressure effects on the non-ideality of the liquid phase.
Conclusions
The esterification of acetic acid with ethanol carried out under CO2 pressure resulted in an increase in product formation compared to the neat reaction. At 333 K and 58.6 bar the CO2 pressure shifted the equilibrium conversion from 63 to 72%.Experimental
The esterification reaction of ethanol and acetic acid was carried out both in neat form and under pressure with CO2. The chemicals used in the esterification reaction for this work are as follows: Acetic acid, ACS grade 99.7% purity, Aldrich Chemical Company Water, HPLC grade, Aldrich Chemical Company Ethanol, Absolute 99.5% purity, Aaper Company Ethyl acetate, HPLC grade 99.8% purity, Aldrich Chemical Company Carbon dioxide, anaerobic grade, Mittler Gas CompanyThe neat reaction, using equimolar amounts of reactants, was allowed to proceed for eight days at 60 °C, at which time the compositions had reached steady values. The compositions at chemical equilibrium were determined by gas chromatographic analysis of samples from the liquid reaction system. GC calibration curves for the quantitative determination of each compound were first determined using a Poropak Q packed column, thermal conductivity detector and helium as the carrier gas.The esterification reaction, using equimolar amounts of the reactants and 30.0 mol% CO2, was performed in CO2 at 58.6 ± 0.5 bar and 60 ± 1 °C. This reaction was measured in a high-pressure variable volume view cell equipped with a quartz window to allow for the visible inspection of the number of phases present (Fig. 4). Two Valco four-port sampling valves allowed for the direct sampling of the top (vapor) and bottom (liquid) phases. Compositions in each phase were determined by injection of isolated liquid and vapor samples into the gas chromatography. Estimated uncertainties in the liquid and gas phase compositions are ±0.005 and ±0.006, respectively. Although samples were drawn from the reaction system, a piston located within the view cell maintained constant pressure within the vessel.
 | ||
| Fig. 4 Variable volume view cell. | ||
Acknowledgements
Financial support from the Environmental Protection Agency (grant R826734-01-0), the National Science Foundation (grant EE697-00537-CRCD), and Clare Booth Luce Fellowship are gratefully acknowledged.References
- B. Subramaniam and M. A. McHugh, Ind. Eng. Chem. Process Des. Dev., 1986, 25, 1 Search PubMed.
- M. J. Burk, S. Feng, M. F. Gross and W. Tumas, J. Am. Chem. Soc., 1995, 117, 8277 CrossRef CAS.
- J. X. Haberman, G. C. Irvin, V. T. John and C. J. Li, Green Chem., 1999, 1, 265 RSC.
- M. McCoy, Chem. Eng. News, 1999, 77((17)), 10 Search PubMed.
- K. Akao and Y. Yoshimura, J. Chem. Phys., 1991, 94, 52433 CrossRef.
- K. P. O’Shea, K. M. Kirmse, M. A. Fox and K. P. Johnston, J. Phys. Chem., 1991, 95, 7863 CrossRef CAS.
- D. L. Tomasko, B. L. Knutson, F. Pouillot, C. L. Liotta and C. A. Eckert, J. Phys. Chem., 1993, 97, 11823 CrossRef CAS.
- Y. Yagi, S. Saito and H. Inomata, J. Chem. Eng. Jpn., 1993, 26, 116 Search PubMed.
- K. Yamasaki and O. Kajimoto, Chem. Phys. Lett., 1990, 172, 271 CrossRef CAS.
- Chemical Synthesis Using Supercritical Fluids, ed. P. G. Jessop and W. Leitner, Wiley-VCH, Weinheim, FRG, 1999. Search PubMed.
- E. E. Reid, Unit Processes in Organic Synthesis, ed. P. H. Groggins, McGraw-Hill Book Co. Inc., NY, 1938. Search PubMed.
- H. Bock, M. Jimoh and G. Wozny, Chem. Eng. Technol., 1997, 20, 182 CAS.
- R. P. Stateva and W. A. Wakeham, Ind. Eng. Chem. Res., 1997, 36, 5474 CrossRef CAS.
- L. S. Lee, W. C. Chen and J. F. Huang, J. Chem. Eng. Jpn., 1996, 29, 427 Search PubMed.
- A. Z. Panagiotopoulos, R. C. Willson and R. C. Reid, J. Chem. Eng. Data, 1988, 33, 321 CrossRef CAS.
- S. Laugier, D. Richon and H. Renon, Fluid Phase Equilibria, 1990, 54, 19 Search PubMed.
- S. Takishima, K. Saiki, K. Arai and S. Saito, J. Chem. Eng. Jpn., 1986, 19, 48 Search PubMed.
- J. H. Yoon, H. Lee and B. H. Chung, Fluid Phase Equilibria, 1994, 102, 287 Search PubMed.
- A. V. Shvarts and G. D. Efremova, Russ. J. Phys. Chem., 1970, 44, 614 Search PubMed.
- A. Zawisza and B. Malesinska, J. Chem. Eng. Data, 1981, 26, 388 CrossRef CAS.
- K. L. Toews, R. M. Shroll, C. M. Wai and N. G. Smart, Anal. Chem., 1995, 67, 4040 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2001 |
