Direct synthesis of organodichlorosilanes by the reaction of metallic silicon, hydrogen chloride and alkene/alkyne and by the reaction of metallic silicon and alkyl chloride
Masaki
Okamoto
,
Satoshi
Onodera
,
Yuji
Yamamoto
,
Eiichi
Suzuki
and
Yoshio
Ono
Department of Chemistry and Materials Science, Tokyo Institute of Technology, Ookayama, Meguro-ku, Tokyo, 152-8550, Japan. Fax: +81-3-5734-2878; E-mail: esuzuki@o.cc.titech.ac.jp
First published on 13th December 2000
Abstract
Dichloroethylsilane was synthesized by the reaction of metallic silicon, hydrogen chloride and ethylene using copper(I) chloride as the catalyst, the silicon conversion and the selectivity for dichloroethylsilane being 36 and 47%, respectively. At a lower reaction temperature or at a higher ratio of ethylene∶hydrogen chloride a higher selectivity was obtained, however the silicon conversion was lower. The silicon–carbon bond formation is caused by the reaction of a surface silylene intermediate with ethylene. The reaction with propylene in place of ethylene gave dichloroisopropylsilane (22% selectivity) and dichloro-n-propyl-silane (8% selectivity) together with chlorosilanes. A part of the dichloroisopropylsilane is formed by the reaction of silicon, hydrogen chloride and isopropyl chloride formed by hydrochlorination of propylene. Use of acetylene instead of alkenes resulted in dichlorovinylsilane formation with a 34% selectivity. Alkyldichlorosilanes were also produced directly from silicon with alkyl chlorides, propyl and butyl chlorides. During the reaction the alkyl chloride is dehydrochlorinated over the surface of copper originating from the catalyst to afford hydrogen chloride and alkene. The hydrogen chloride formed participates in the formation of the silicon–hydrogen bond in alkyldichlorosilane, and the reaction of silicon, hydrogen chloride and alkene also causes alkyldichlorosilane formation. The reaction with isopropyl chloride gave a very high selectivity (85%) for dichloroisopropylsilane, the silicon conversion being 86%.
Introduction
Dichlorodimethylsilane is industrially produced in the largest scale in the silicon industry as a raw material for synthesizing silicone polymers.1,2 Methylchlorosilanes, mainly dichlorodimethylsilane, are obtained by reaction of metallic silicon with methyl chloride using a copper catalyst.1,2 To yield organohalogenosilanes directly from metallic silicon commercially, only organic halides, such as methyl chloride and chlorobenzene, have been used as a reactant.Hydrogen chloride also reacts with metallic silicon to afford trichlorosilane as a main product together with dichlorosilane and tetrachlorosilane.3 We have found that the reaction of metallic silicon and hydrogen chloride in the presence of ethylene affords ethylchlorosilanes, mainly dichloroethylsilane, together with chlorosilanes.4 Furthermore, when acetylene was used instead of ethylene dichlorovinylsilane was obtained.4 This is a new reaction for a direct synthesis of organochlorosilanes.
To explain trichlorosilane formation as a main product in the Si–HCl reaction and dichloroethylsilane formation in the Si–HCl–C2H4 reaction, we have proposed a mechanism involving surface silylene intermediate![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 4 as shown in eqns. (1) and (2), respectively. In the Si–HCl reaction (eqn. 1) a silylene 1 is formed on the silicon surface. Hydrogen chloride reacts with 1 to afford chlorosilicon species 2. The addition of hydrogen chloride to dichlorosilylene to give trichlorosilane has been reported.5 Attack of hydrogen chloride on species 2 leads to formation of dichlorosilicon species 3, which is subsequently attacked by hydrogen chloride and two Si–Cu bonds are simultaneously cleaved to give trichlorosilane. In the presence of ethylene (eqn. 2) the silylene intermediate 1 reacts to form silacyclopropane species 4. It is well known that a silylene intermediate adds to an alkene to give a vinylsilane via a silacyclopropane intermediate.6,7 Then, species 4 is ring-cleaved by attack of hydrogen chloride, and hydrogen chloride attacks surface species 5 consequently to form dichloroethylsilane. We have also reported the intermediacy of surface silylene 1 in the reaction of silicon with methanol.8 Thus, adding ethylene to the methanol feed resulted in the formation of ethyldimethoxysilane via1.8,9
4 as shown in eqns. (1) and (2), respectively. In the Si–HCl reaction (eqn. 1) a silylene 1 is formed on the silicon surface. Hydrogen chloride reacts with 1 to afford chlorosilicon species 2. The addition of hydrogen chloride to dichlorosilylene to give trichlorosilane has been reported.5 Attack of hydrogen chloride on species 2 leads to formation of dichlorosilicon species 3, which is subsequently attacked by hydrogen chloride and two Si–Cu bonds are simultaneously cleaved to give trichlorosilane. In the presence of ethylene (eqn. 2) the silylene intermediate 1 reacts to form silacyclopropane species 4. It is well known that a silylene intermediate adds to an alkene to give a vinylsilane via a silacyclopropane intermediate.6,7 Then, species 4 is ring-cleaved by attack of hydrogen chloride, and hydrogen chloride attacks surface species 5 consequently to form dichloroethylsilane. We have also reported the intermediacy of surface silylene 1 in the reaction of silicon with methanol.8 Thus, adding ethylene to the methanol feed resulted in the formation of ethyldimethoxysilane via1.8,9
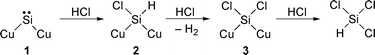
| (1) |
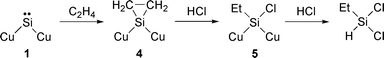
| (2) |
In this work the reaction of metallic silicon, hydrogen chloride and an unsaturated hydrocarbon, ethylene, propylene or acetylene, was carried out for synthesis of alkyl- or vinyl-dichlorosilane, and the effects of variables and the reaction pathway were examined. Propylchlorosilanes and butylchlorosilanes can be prepared from metallic silicon and propyl and butyl chlorides, respectively, the main products being alkyldichlorosilanes, not dialkyldichlorosilane.10 This is different from the product distribution of methylchlorosilane formation in the Si–CH3Cl reaction, which gives dichlorodimethylsilane as a main product. The alkyldichlorosilane formed in the reaction of silicon with propyl or butyl chloride is the same as the main product![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 4 in the Si–HCl–alkene reaction. This suggests that alkyldichlorosilanes are formed by the reaction of silicon, hydrogen chloride and propylene/butene formed by dehydrochlorination of propyl or butyl chloride. In this work the reactions of silicon with propyl and butyl chlorides were carried out, and the mechanism of alkyldichlorosilane formation was examined.
4 in the Si–HCl–alkene reaction. This suggests that alkyldichlorosilanes are formed by the reaction of silicon, hydrogen chloride and propylene/butene formed by dehydrochlorination of propyl or butyl chloride. In this work the reactions of silicon with propyl and butyl chlorides were carried out, and the mechanism of alkyldichlorosilane formation was examined.
Experimental
Silicon grains (45–63 μm, purity 99.9%, impurities Fe 0.008 wt%, Sb 0.003 wt%) were washed by a 46% hydrogen fluoride aqueous solution for 1 h at room temperature to remove SiO2 overlayers. The grains (7.1 or 8.9 mmol) and copper(I) chloride grains (45–63 μm, purity 99.9%, Cu/(CuCl + Si) = 2.5–40 wt%) as a catalyst were physically mixed, placed in a quartz tube (i.d. 10 mm) in a fixed-bed flow reactor system and preheated at 723 K for 10 min in a helium stream. A mixture of hydrogen chloride (3–15 mmol h−1) and alkene/alkyne (5–12 mmol h−1) gases was fed to the reactor at 553–723 K. The reactor effluents were analysed by a gas chromatograph every 10 min. The products were identified by 1H NMR and GC–MS. In the reaction of silicon with alkyl chloride the flow rates of alkyl chloride and helium were 5 and 10 mmol h−1, respectively. Selectivity (%) = 100 × amount of product (mmol)/sum of amounts of silicon-containing products (mmol); yield (%) = 100 × amount of product (mmol)/amount of silicon charged in the reactor (7.1 or 8.9 mmol); silicon conversion (%) = 100 × sum of amounts of silicon-containing products (mmol)/amount of silicon charged in the reactor (7.1 or 89 mmol).Results and discussion
Dichloroethylsilane synthesis from metallic silicon, hydrogen chloride and ethylene
The reaction of silicon, hydrogen chloride and ethylene was carried out at various temperatures, after preheating a mixture of silicon and copper(I) chloride grains in a helium stream at 723 K for 10 min. Fig. 1 shows the changes of the formation rates with time at 493, 513 and 533 K. Dichloroethylsilane was formed together with trichlorosilane, dichlorosilane and tetrachlorosilane. At 533 K all the formation rates immediately increased after starting to feed the mixture of hydrogen chloride and ethylene to the reactor, and were almost constant after 20 min. An induction time was observed below 513 K and lengthened with decreasing reaction temperature. The rate of trichlorosilane formation at the steady period decreased with decrease of reaction temperature, while the rate of formation of dichloroethylsilane did not change. The effect of reaction temperature on the overall selectivity for ethyldimethoxysilane and the silicon conversion for 5 h is shown in Fig. 2. The selectivity for dichloroethylsilane was higher at a lower temperature, while the silicon conversion was lower.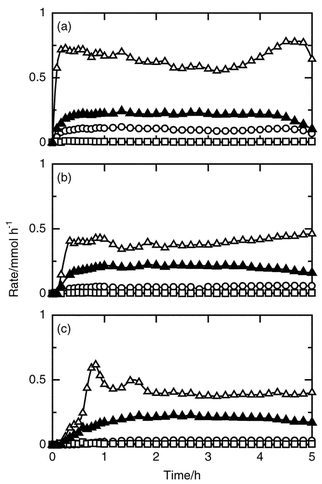 | ||
| Fig. 1 Time courses of the formation rates of dichloroethylsilane (filled triangle), dichlorosilane (open circle), trichlorosilane (open triangle) and tetrachlorosilane (open square) in the reaction of silicon, hydrogen chloride and ethylene at 533 (a), 513 (b) and 493 K (c). Catalyst amount was 10 wt%, amount of silicon charged in the reactor 8.9 mmol, pretreatment at 723 K for 10 min, flow rates of hydrogen chloride and ethylene 5 and 10 mmol h−1, respectively. | ||
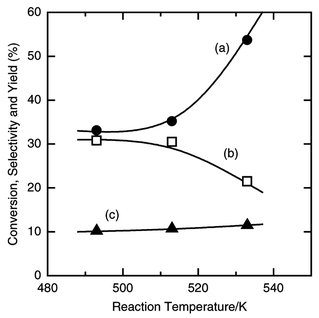 | ||
| Fig. 2 Effect of reaction temperature on the silicon conversion (a), the selectivity (b) and the yield of dichloroethylsilane (c) in the reaction of silicon, hydrogen chloride and ethylene. The pretreatment and reaction conditions are the same as those in Fig. 1. The reaction time was 5 h. | ||
The ratio of ethylene to hydrogen chloride was varied. The results for 5 h are shown in Fig. 3. The selectivity for dichloroethylsilane increased with the ratio and reached 39% at 4∶1. With decreasing ratio the silicon conversion increased to 80% at 0.5∶1. The reaction rate had fallen to zero at 4 h with an ethylene to hydrogen chloride ratio of 0.5∶1, whereas with a ratio of 2 or 5∶1, the reaction was still observed after 5 h. At a ratio of 4∶1 and a reaction time of 12 h, 36% of the silicon charged in the reactor had been converted and the selectivity for dichloroethylsilane was 47%. The silicon conversion must be larger when the reaction time is prolonged.
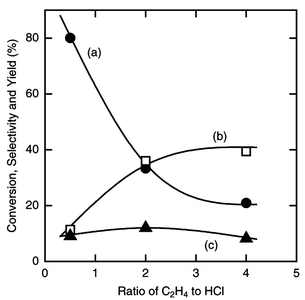 | ||
| Fig. 3 Effect of the ratio of ethylene to hydrogen chloride on the silicon conversion (a), the selectivity (b) and the yield of dichloroethylsilane (c) in the reaction of silicon, hydrogen chloride and ethylene. Catalyst amount 10 wt%, amount of silicon charged 8.9 mmol, pretreatment carried out at 723 K for 10 min, total flow rate 15 mmol h−1, reaction temperature 513 K, reaction time 5 h. | ||
Dichloropropylsilane synthesis from silicon, hydrogen chloride and propylene
Instead of ethylene, propylene was fed with hydrogen chloride. The time courses of the formation rates are shown in Fig. 4. Dichloroisopropylsilane and dichloro-n-propylsilane were formed as organosilanes together with dichlorosilane, trichlorosilane and tetrachlorosilane. The formation rate of dichloroisopropylsilane was almost twice as high as that of the n-propyl product. The reaction stopped at 9.5 h and the silicon conversion reached 61%. The overall selectivities for dichloroisopropylsilane and dichloro-n-propylsilane were 22 and 8%, respectively.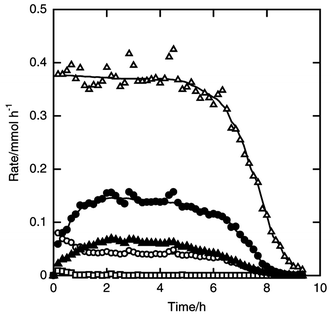 | ||
| Fig. 4 Time courses of the formation rates of dichloroisopropylsilane (filled circle), dichloro-n-propylsilane (filled triangle), dichlorosilane (open circle), trichlorosilane (open triangle) and tetrachlorosilane (open square) in the reaction of silicon, hydrogen chloride and propylene. Catalyst amount 3 wt%, amount of silicon charged 7.1 mmol, pretreatment carried out at 823 K for 10 min, flow rates of hydrogen chloride and propylene 3 and 12 mmol h−1, reaction temperature 553 K. | ||
The effect of reaction temperature was examined. In Table 1 the results for 5 h reaction are summarized. At 513 and 493 K the reaction still proceeded at 5 h, while at 573, 553 and 533 K the reaction rates were almost zero at 3, 3 and 5 h, respectively. Above 533 K the silicon conversion reached around 90%. At 553 K the highest selectivity for dichloroisopropylsilane was obtained. The ratio of the iso to the n product changed with temperature, and 7.3∶1 at 533 K was the highest.
| Selectivity (%) | |||||||
|---|---|---|---|---|---|---|---|
| Reaction temperature/K | Silicon conversion (%) | (iso-C3H7)HSiCl2 | (n-C3H7)HSiCl2 | H2SiCl2 | HSiCl3 | SiCl4 | Mole ratio iso∶n |
| Catalyst amount 5 wt%, amount of silicon charged in the reactor 7.1 mmol, pretreatment carried out at 723 K for 10 min, flow rates of hydrogen chloride and propylene 10 and 5 mmol h−1, respectively. | |||||||
| 573 | 94 | 9 | 3 | 13 | 74 | 1 | 3.2∶1 |
| 553 | 87 | 13 | 3 | 10 | 73 | 1 | 5.0∶1 |
| 533 | 90 | 7 | 1 | 6 | 84 | 2 | 7.3∶1 |
| 513 | 64 | 5 | 1 | 3 | 87 | 4 | 5.8∶1 |
| 493 | 19 | 2 | 1 | 1 | 86 | 10 | 4.0∶1 |
The ratio of propylene to hydrogen chloride was changed, while the total flow rate was maintained at 15 mmol h−1, the results being shown in Table 2. The conversion was higher at a lower ratio and was 87% at 0.5∶1. Both the selectivities for iso- and n-propyldichlorosilanes increased with increase of the ratio. The ratio of iso∶n product also changed. Under HCl-rich conditions the product ratio was high.
| Selectivity (%) | |||||||
|---|---|---|---|---|---|---|---|
| Mole ratio C3H6∶HCl | Silicon conversion (%) | (iso-C3H7)HSiCl2 | (n-C3H7)HSiCl2 | H2SiCl2 | HSiCl3 | SiCl4 | Mole ratio iso∶n |
| Catalyst amount 5 wt%, the amount of silicon charged 7.1 mmol, pretreatment at 723 K for 10 min, reaction temperature 553 K, total flow rate 15 mmol h−1. | |||||||
| 4∶1 | 60 | 19 | 8 | 9 | 64 | 0 | 2.4∶1 |
| 2∶1 | 75 | 17 | 5 | 9 | 68 | 1 | 3.4∶1 |
| 0.5∶1 | 87 | 13 | 3 | 10 | 73 | 1 | 5.0∶1 |
The optimum conditions for a high selectivity for dichloroisopropylsilane were determined. A 22% selectivity for dichloroisopropylsilane and an 8% selectivity for dichloro-n-propylsilane were obtained at 553 K at 12 mmol h−1 of propylene and 3 mmol h−1 of hydrogen chloride using 3 wt% copper catalyst after pretreatment at 773 K for 10 min, the silicon conversion being 61%.
A plausible mechanism for propyldichlorosilanes formation is shown in eqn. (3). The reaction of surface silylene 1 with propylene gives a methylsilacyclopropane species 6, which is attacked by hydrogen chloride. When bond a in 6 is cleaved a n-propylchlorosilicon species is formed and finally dichloro-n-propylsilane is obtained. On the other hand, cleavage of bond b gives dichloroisopropylsilane.
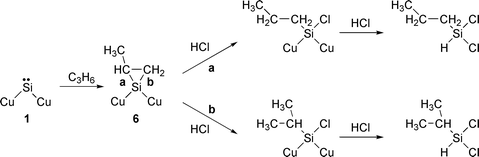
| (3) |
In the reaction of silicon, hydrogen chloride and propylene, ca. 0.3 mmol h−1 of isopropyl chloride was formed as a by-product by hydrochlorination of propylene. It has been reported that isopropyl chloride reacts with metallic silicon![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 10 and that, in the presence of hydrogen chloride, dichloromethylsilane is formed in the reaction of silicon with methyl chloride for the synthesis of dichlorodimethylsilane.11 It is highly possible that the isopropyl chloride formed during the Si–HCl–C3H6 reaction also contributes to the dichloroisopropylsilane formation.
10 and that, in the presence of hydrogen chloride, dichloromethylsilane is formed in the reaction of silicon with methyl chloride for the synthesis of dichlorodimethylsilane.11 It is highly possible that the isopropyl chloride formed during the Si–HCl–C3H6 reaction also contributes to the dichloroisopropylsilane formation.
Between 1 and 2 h, 2 mmol h−1 of isopropyl chloride was added to the feed of the HCl–C3H6 mixture. Fig. 5 shows the changes of formation rates with time. Just after adding isopropyl chloride, only the rate of formation of dichloroisopropylsilane increased. This strongly indicates that isopropyl chloride participates in the formation of dichloroisopropylsilane. As shown in eqn. (4), isopropyl chloride reacts with surface silylene 1 to afford the surface species 7, which is converted into dichloroisopropylsilane by attack of hydrogen chloride.
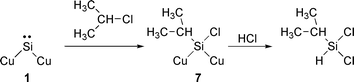
| (4) |
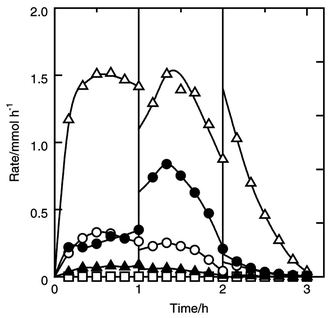 | ||
| Fig. 5 Effect of isopropyl chloride addition on the formation rates of the products in the reaction of silicon, hydrogen chloride and propylene. Dichloroisopropylsilane (filled circle), dichloro-n-propylsilane (filled triangle), dichlorosilane (open circle), trichlorosilane (open triangle) and tetrachlorosilane (open square). Catalyst amount 5 wt%, amount of silicon charged in 7.1 mmol, pretreatment at 723 K for 10 min, flow rates of hydrogen chloride and propylene 10 and 5 mmol h−1, flow rate of isopropyl chloride added between 1 and 2 h 2 mmol h−1, reaction temperature 553 K. | ||
Dichlorovinylsilane synthesis from silicon, hydrogen chloride and acetylene
A mixture of silicon and copper(I) chloride was preheated in a helium stream at 723 K for 10 min. First, only hydrogen chloride was fed to the reactor at 513 K for 10 min to activate the silicon–catalyst mixture before feeding a mixture of hydrogen chloride and acetylene, because the reaction did not proceed without activation by hydrogen chloride. During the activation period 1% of the silicon charged was consumed to form chlorosilanes, mainly trichlorosilane. After the feed was changed to the mixture of hydrogen chloride and acetylene, dichlorovinylsilane and dichloroethylsilane were formed together with chlorosilanes.3Fig. 6 shows the time courses of the formation rates. The rate curves of dichlorovinylsilane and trichlorosilane were very similar. Before 2 and 4 h, the rate of formation of dichloroethylsilane was almost same as those of dichlorovinylsilane and trichlorosilane, while around 2.5 h it was half that of dichlorovinylsilane. The silicon conversion was 13% for 5 h, the overall selectivities of dichlorovinylsilane, dichloroethylsilane and trichlorosilane being 39, 23 and 38%, respectively. Trace amounts of dichlorosilane and tetrachlorosilane were formed.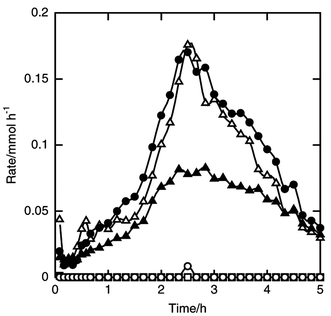 | ||
| Fig. 6 Time courses of the formation rates of dichlorovinylsilane (filled circle), dichloroethylsilane (filled triangle), dichlorosilane (open circle), trichlorosilane (open triangle) and tetrachlorosilane (open square) in the reaction of silicon, hydrogen chloride and acetylene. Catalyst amount 1 wt%, amount of silicon charged 8.9 mmol, pretreatment at 723 K for 10 min, silicon–catalyst mixture activated by 15 mmol h−1 of hydrogen chloride at 513 K for 10 min, flow rates of hydrogen chloride and acetylene 15 and 4 mmol h−1, respectively, reaction temperature 513 K. | ||
The ratio of acetylene to hydrogen chloride was varied. The silicon conversion and the product distribution greatly depended on the ratio, as described in our previous communication.4 Without acetylene, trichlorosilane was mainly formed, and the silicon conversion was very high, 85%. On increasing the ratio the conversion decreased, while the selectivity for dichlorovinylsilane increased and reached 34% at 0.26∶1.
The effect of reaction temperature is summarized in Table 3. The silicon conversion increased with reaction temperature till 523 K. Both the selectivities for dichlorovinylsilane and dichloroethylsilane were higher at a lower temperature and reached 36 and 26% at 493 K, respectively.
| Selectivity (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reaction temperature/K | Silicon conversion (%) | (C2H3)HSiCl2 | (C2H5)HSiCl2 | H2SiCl2 | HSiCl3 | SiCl4 | ||
| Catalyst amount 5 wt%, amount of silicon charged 8.9 mmol, pretreatment at 723 K for 10 min, flow rates of hydrogen chloride and acetylene 15 and 4 mmol h−1, respectively. | ||||||||
| 533 | 24 | 18 | 10 | 8 | 63 | 1 | ||
| 523 | 29 | 29 | 16 | 4 | 50 | 1 | ||
| 513 | 16 | 34 | 21 | 0 | 45 | 0 | ||
| 503 | 13 | 31 | 18 | 1 | 49 | 1 | ||
| 493 | 8 | 36 | 26 | 0 | 38 | 0 | ||
Eqn. (5) shows the mechanism of formation of dichlorovinylsilane and dichloroethylsilane. The reaction of surface silylene 1 with acetylene gives a silacyclopropene species 8, which is ring-cleaved by attack of hydrogen chloride. The formed vinylchlorosilicon species 9 is finally converted into dichlorovinylsilane. Normally a gas-phase silylene reacts with acetylene to afford 1,4-disilacyclohexa-2,5-diene, which is a double-added product.7,12 No formation of 1,4-disilacyclohexa-2,5-diene in the Si–HCl–C2H2 reaction indicates that the silylene species is not present in the gas phase, but on the surface. During the Si–HCl–C2H2 reaction vinyl chloride was not formed by hydrochlorination of acetylene. This shows that vinyl chloride does not participate in the dichlorovinylsilane formation. Additionally, under the reaction conditions, the hydrogenation of acetylene or dichlorovinylsilane by hydrogen formed as a by-product did not proceed. This strongly suggests that dichloroethylsilane is formed neither by the reaction of surface silylene with ethylene nor by hydrogenation of dichlorovinylsilane and that the vinyl group in species 9 is not converted into an ethyl group. Thus, it was concluded that hydrogenation of the surface intermediate 8 occurs to form dichloroethylsilane.
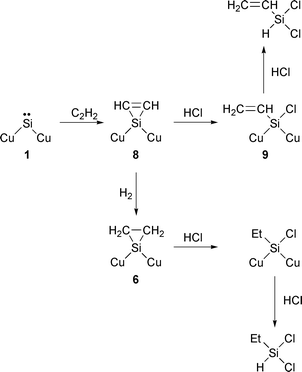
| (5) |
Alkyldichlorosilane synthesis from silicon and alkyl chloride
Petrov et al. have reported that propylchlorosilanes and butylchlorosilanes were directly synthesized by the reaction of silicon with propyl and butyl chlorides, respectively, and that the corresponding alkyldichlorosilanes were mainly formed among organosilanes.10 In the reaction of silicon with alkyl chloride it is possible that the reaction of silicon with hydrogen chloride and alkene coming from alkyl chloride also causes the formation of alkyldichlorosilane since the main product is the same as that in the Si–HCl–alkene reaction. The synthesis of alkyldichlorosilanes was performed by the reaction of silicon with various alkyl chlorides, and the mechanism of alkyldichlorosilane formation was examined.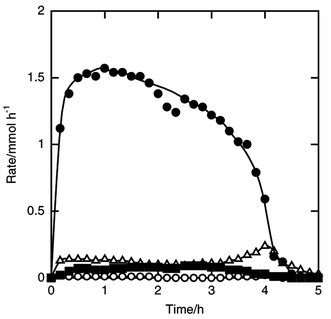 | ||
| Fig. 7 Time courses of the formation rates of dichloroisopropylsilane (filled circle), trichloroisopropylsilane (filled square), dichlorosilane (open circle) and trichlorosilane (open triangle) in the reaction of silicon and isopropyl chloride. Catalyst amount 5 wt%, amount of silicon charged 7.1 mmol, pretreatment at 723 K for 10 min, flow rates of isopropyl chloride and helium 5 and 10 mmol h−1, reaction temperature was 573 K. | ||
On the other hand, the reaction with n-propyl chloride gave a very low silicon conversion at 5 h, 2%. Dichloro-n-propylsilane was selectively formed with a 98% selectivity. n-Propyl chloride hardly decomposed to form propylene and hydrogen chloride, the amount of propylene formed being below 0.2 mmol h−1. This probably accounts for the low silicon conversion, because hydrogen chloride is necessary for dichloropropylsilane formation in the last step, which is rate-determining.
| Selectivity (%) | |||||
|---|---|---|---|---|---|
| Butyl chloride | Silicon (%) | (C4H9)HSiCl2 | (C4H9)SiCl3 | HSiCl3 | SiCl4 |
| The parentheses represent the kinds of butyl group in butyldichlorosilanes or butyltrichlorosilanes. Catalyst amount 5 wt%, amount of silicon charged 7.1 mmol, pretreatment at 723 K for 10 min, flow rates of helium and butyl chloride 10 and 5 mmol h−1, respectively. | |||||
| n | 29 | 68 (n) | 3 (n) | 18 | 0 |
| 11 (sec) | |||||
| iso | 66 | 39 (tert) | 1 (tert) | 40 | 2 |
| 18 (iso) | |||||
| sec | 82 | 16 (sec) | 0 | 79 | 5 |
| tert | 79 | 3 (tert) | 0 | 81 | 16 |
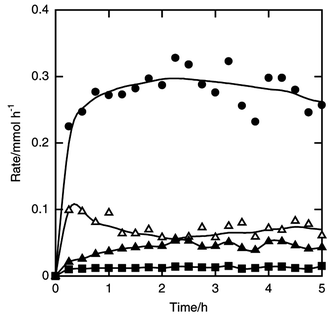 | ||
| Fig. 8 Time courses of the formation rates of n-butyldichlorosilane (filled circle), n-butyltrichlorosilane (filled square), sec-butyldichlorosilane (filled triangle) and trichlorosilane (open triangle) in the reaction of silicon and n-butyl chloride. Catalyst amount 5 wt%, amount of silicon charged 7.1 mmol, pretreatment at 723 K for 10 min, flow rates of n-butyl chloride and helium 5 and 10 mmol h−1, reaction temperature 573 K. | ||
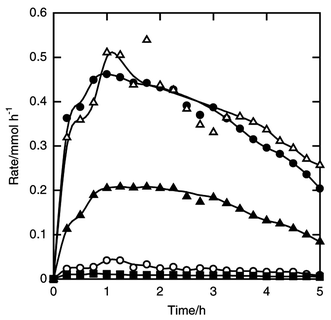 | ||
| Fig. 9 Time courses of the formation rates of tert-butyldichlorosilane (filled circle), tert-butyltrichlorosilane (filled square), isobutyldichlorosilane (filled triangle), dichlorosilane (open circle) and trichlorosilane (open triangle) in the reaction of silicon and isobutyl chloride. Flow rates of isobutyl chloride and helium 5 and 10 mmol h−1, respectively. Other details as in Fig. 8. | ||
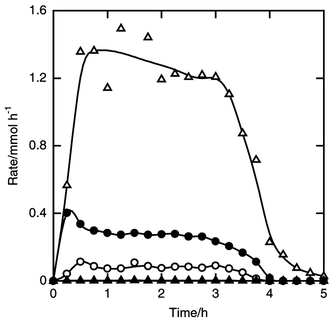 | ||
| Fig. 10 Time courses of the formation rates of sec-butyldichlorosilane (filled circle), sec-butyltrichlorosilane (filled triangle), dichlorosilane (open circle) and trichlorosilane (open triangle) in the reaction of silicon and sec-butyl chloride. Flow rates of sec-butyl chloride and helium 5 and 10 mmol h−1. Other details as in Fig. 8. | ||
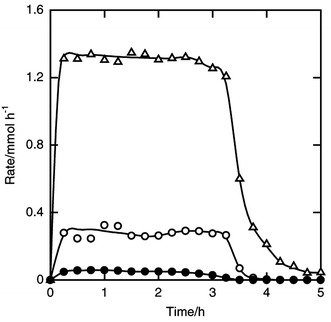 | ||
| Fig. 11 Time courses of the formation rates of tert-butyldichlorosilane (filled circle), dichlorosilane (open circle) and trichlorosilane (open triangle) in the reaction of silicon and tert-butyl chloride. Flow rates of tert-butyl chloride and helium 5 and 10 mmol h−1. Other details as in Fig. 8. | ||
Ishikawa et al. have reported the reaction of phenyl(trimethylsilyl)silylene with alkyl chlorides.13 The reaction with n-octyl chloride resulted in carbon–chlorine insertion of the silylene to form 1-chloro-1-octyl-1-phenyltrimethyldisilane with 9% yield. sec-Butyl chloride also gave the carbon–chlorine insertion product (7% yield) together with 1-chloro-2,2,2-trimethyl-1-phenyldisilane (10% yield), 1-butene (7% yield) and trans-2-butene (5% yield). They proposed a mechanism for butene formation that involves a zwitterion intermediate 10 shown in eqn. (6). When reaction a proceeds the insertion product is formed. Reaction b leads to formation of butene and hydrodisilane.
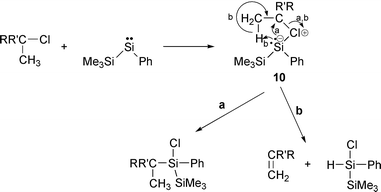
| (6) |
In the case of tert-butyl chloride, no silylene insertion product was detected, and 1-chloro-2,2,2-trimethyl-1-phenyldisilane and isobutene were formed with 27 and 28% yields, respectively. These results show that the tendency to the elimination of alkene decreases for the zwitterion intermediate formed from tert- > sec- > n-alkyl chloride. The reactivity of alkyl chloride to a silylene intermediate can be determined by comparing the total yield of the insertion and elimination products. Thus, the order of the reactivity of alkyl chloride is tert > sec > n.
The study of β-hydride elimination of various alkyl groups formed on a copper crystal by corresponding alkyl chlorides has been reported.14sec- and tert-Butyl groups more easily decompose to form butenes than n-butyl. This strongly suggests that decomposition of sec- and tert-butyl chloride occurs easily over a copper surface to give butene and hydrogen chloride.
Taking account of these literature results,13,14 in the reactions of silicon with butyl chlorides it is highly plausible that formation of butenes is caused both by the butyl chloride decomposition over the copper surface and by decomposition of a zwitterion intermediate formed by the reaction of surface silylene and butyl chloride. In the reactions of silicon with butyl chlorides sec- and tert-butyl chlorides resulted in 100% conversions of butyl chloride, and the formation rates of butenes were 4.5 and 3.5 mmol h−1, respectively, while iso- and n-butyl chloride were not completely converted. The amounts of butenes formed from iso- and n-butyl chloride were 2.5 and 1.0 mmol h−1, respectively. These results are compatible with both the zwitterion stability and the butyl chloride stability over copper metal.
The reaction mechanism is shown in eqn. (7). Butyl chloride reacts with surface silylene 1 to form surface species 11. If the reaction a proceeds butyldichlorosilane is formed via intermediate 12. Reaction b leads to the formation of butene and species 2, the latter reacting with hydrogen chloride originating from butyl chloride to give trichlorosilane finally. Among various butyl chlorides, tert-butyl chloride gave the highest selectivity for trichlorosilane. This result is in agreement with the highest yield of hydrodisilane in the reaction of phenyl(trimethylsilyl)silylene with tert-butyl chloride and with easy formation of hydrogen chloride from tert-butyl chloride over the copper surface. Comparing the silicon conversion for 5 h in the reactions of silicon with various butyl chlorides, use of n-butyl chloride resulted in the lowest silicon conversion, because it is not easy to form hydrogen chloride by decomposition of butyl chloride over copper.
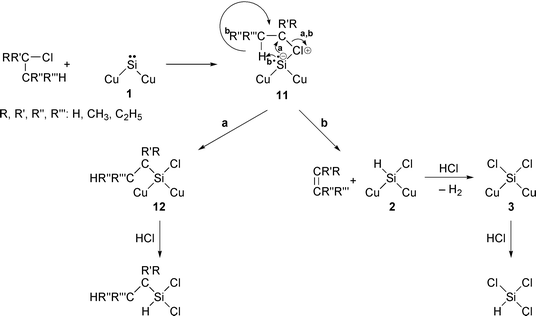
| (7) |
The reaction with n-butyl chloride resulted in the formation of sec-butyldichlorosilane together with n-butylchlorosilanes. The sec-butyldichlorosilane formation may be caused by the reaction of silicon, hydrogen chloride and 1-/2-butene formed from n-butyl chloride and/or by the reaction of silicon, hydrogen chloride and sec-butyl chloride formed by isomerization of n-butyl chloride. Actually, the formation of ca. 0.2 mmol h−1 of sec-butyl chloride was observed during the reaction.
In the case of isobutyl chloride, tert-butyldichlorosilane was mainly formed with a 39% selectivity, and the selectivity for isobutyldichlorosilane was about a half (18%) that for the tert-butyldichlorosilane. Since the isobutyl group is primary a smaller amount of hydrogen chloride is probably formed than that using sec- or tert-butyl chloride. This causes a lower silicon conversion. The formation rate of tert-butyl chloride was below 0.02 mmol h−1, indicating no reaction of silicon, hydrogen chloride and tert-butyl chloride to afford tert-butyldichlorosilane. Thus, isobutene and hydrogen chloride formed from isobutyl chloride take part in the formation of tert-butyldichlorosilane.
Taken in total, it is concluded that alkyldichlorosilane is formed both by the reaction of silicon, hydrogen chloride and alkyl chloride and by the reaction of silicon, hydrogen chloride and alkene.
Conclusion
Alkyl- or vinyl-dichlorosilane was synthesized by the reaction of silicon, hydrogen chloride and alkene/alkyne. A silicon–carbon bond is formed mainly by the reaction of a surface silylene intermediate with alkene or alkyne. During the reaction a small part of the alkene undergoes hydrochlorination to yield alkyl chloride, which subsequently reacts with the surface silylene to form the silicon–carbon bond. The reaction of silicon with propyl or butyl chloride also gave alkyldichlorosilane as a main organosilicon product. Alkyl chloride is dehydrochlorinated over the copper surface, and hydrogen chloride formed participates in the formation of the silicon–hydrogen bond. Alkyldichlorosilane is formed not only by the reaction of silicon, hydrogen chloride and alkyl chloride but by the reaction of silicon, hydrogen chloride and alkene.References
- R. J. H. Voorhoeve , Organohalosilanes: Precursors to Silicones, Elsevier, Amsterdam, 1967. Search PubMed.
- K. M. Lewis , D. McLeod , B. Kanner , J. L. Falconer and T. Frank , in Catalyzed Direct Reactions of Silicon, eds. K. M. Lewis and D. G. Rethwisch, Elsevier, Amsterdam, 1993, pp. 333–440 and references therein. Search PubMed.
- I. Shiihara and J. Iyoda, Bull. Chem. Soc. Jpn., 1959, 32, 636 CAS; K. M. Lewis , D. McLeod , B. Kanner , J. L. Falconer and T. Frank , in Catalyzed Direct Reactions of Silicon, eds. K. M. Lewis and D. G. Rethwisch, Elsevier, Amsterdam, 1993, pp. 1–66 and references therein; Search PubMed; K. M. Lewis , D. McLeod , B. Kanner , J. L. Falconer and T. Frank , in Catalyzed Direct Reactions of Silicon, eds. K. M. Lewis and D. G. Rethwisch, Elsevier, Amsterdam, 1993, pp. 441–457. Search PubMed.
- M. Okamoto, S. Onodera, Y. Yamamoto, E. Suzuki and Y. Ono, Chem. Commun., 1998, 1275 RSC.
- R. C. Bracken , U.S. Pat., 3 565 590, 1971. .
- P. S. Skell and E. J. Goldstein, J. Am. Chem. Soc., 1964, 86, 1442; V. J. Tortorelli, M. Jones, Jr., S. Wu and Z. Li, Organometallics, 1983, 2, 759 CrossRef CAS; M. Ishikawa, K. Nakagawa and M. Kumada, J. Organomet. Chem., 1979, 178, 105 CrossRef CAS.
- P. P. Gaspar , in Reactive Intermediates, eds. M. Jones, Jr. and R. A. Moss, Wiley, New York, 1981, vol. 2, pp. 335–385 and references therein; Search PubMed; Y.-N. Tang , in Reactive Intermediates, ed. R. A. Abramovitch, Plenum Press, New York, 1982, vol. 2, pp. 297–366 and references therein. Search PubMed.
- M. Okamoto, E. Suzuki and Y. Ono, J. Catal., 1994, 145, 537 CrossRef CAS.
- M. Okamoto, N. Watanabe, E. Suzuki and Y. Ono, J. Organomet. Chem., 1995, 489, C12 CrossRef CAS; M. Okamoto , J. Komai , M. Uematsu , E. Suzuki and Y. Ono , J. Organomet. Chem., in the press. Search PubMed.
- A. D. Petrov, N. P. Smetankina and G. I. Nikishin, J. Gen. Chem. USSR, 1955, 25, 2305 Search PubMed.
- R. L. Halm and R. H. Zapp , U. S. Pat., 4 966 986, 1990. Search PubMed.
- T. J. Barton and J. A. Kilgour, J. Am. Chem. Soc., 1974, 96, 7150 CrossRef CAS; E. A. Chernyshev, N. G. Kommalenkova, S. A. Bashkirova and V. V. Sokolov, Zh. Obshch. Khim., 1978, 48, 830 Search PubMed; E. A. Chernyshev, N. G. Kommalenkova and S. A. Bashkirova, Zh. Obshch. Khim., 1971, 41, 1175 Search PubMed; M. Ishikawa, K. Nakagawa and M. Kumada, J. Organomet. Chem., 1977, 131, C15 CrossRef CAS; W. H. Atwell and D. R. Weyenberg, J. Am. Chem. Soc., 1968, 90, 3438 CrossRef CAS.
- M. Ishikawa, K. Nakagawa, S. Katayama and M. Kumada, J. Organomet. Chem., 1981, 216, C48 CrossRef CAS.
- J.-L. Lin, A. V. Teplyakov and B. E. Bent, J. Phys. Chem., 1996, 100, 110721.
| This journal is © The Royal Society of Chemistry 2001 |
