Immunodetection of lactosylated proteins as a useful tool to determine heat treatment in milk samples
Micaela Pallinia, Dario Compagnonea, Stefania Di Stefanob, Stefano Marinib, Massimiliano Colettab and Giuseppe Palleschia
aDipartimento di Scienze e Tecnologie Chimiche, Università di Roma ‘Tor Vergata’, Via della Ricerca Scientifica, 00133, Rome, Italy
bDipartimento di Medicina Sperimentale e Scienze
Biochimiche, Università di Roma ‘Tor Vergata’, Via di Tor Vergata, 00133, Rome, Italy
First published on 1st December 2000
Abstract
This paper reports the optimisation of a competitive immunoassay (ELISA) to detect lactosylated proteins in milk samples. The assay employs monoclonal antibodies for lactosylated proteins produced in our laboratory and requires no pre-treatment of the samples other than a dilution step. Monoclonal antibodies were fully characterised in terms of selectivity and cross-reactivity with structurally related molecules and used in a competitive assay format with lactosylated standard proteins (lactosylated ovalbumin). The detection limit for lactosylated ovalbumin was 0.015 μg ml−1 and the working range was from 0.010 to 40 μg ml−1. The data obtained indicate that the ELISA developed is applicable to diluted milk samples and is able to distinguish between milk samples that have undergone different heat treatments (UHT and pasteurised milk).
Introduction
Heat treatment of milk samples during pasteurisation processes leads to the non enzymatic lactosylation of free amino groups of proteins due to the Maillard reaction.1 The addition of lactose to lysine ε-amino groups forms N-substituted-1-amino-deoxy-2-ketoses, known as Amadori compounds, subsequently leading to unavailable amino acid sugar reaction products.2,3 The blocked lysine will not be susceptible to further enzyme attack, becoming nutritionally unavailable. Since lysine is an essential amino acid, it is not synthesised by humans, and it must be present in the diet. Hence, the main consequence of these heating processes is the modification of the nutritional values of milk.The two main heat induced markers used as indicators of the severity of heat treatment are furosine and lactulose. Furosine, namely ε-N-(-2-furoylmethyl)-L-lysine, is an amino acid obtained by acid hydrolysis of glycosylated proteins4 and is mainly detected by reversed-phase HPLC5 or ion-exchange chromatography,6 although lately capillary electrophoresis has also been reported.7 Hydrolysis of lactulosyllysine, the lysine–lactose adduct formed in MR, yields three different products, lysine (∼50%), furosine (∼30–40%) and pyridosine (∼10–20%), but the conversion factor for the calculation of the content of the Amadori product is uncertain since the proportions of the hydrolysis products are variable. Microwave hydrolysis of proteins has been applied to furosine determination, shortening the time required for the whole procedure.8 The furosine method has the advantage of being sensitive at very low levels of blocked lysine, but the overall procedure, consisting of 24 h hydrolysis and chromatographic determination, is cumbersome and time consuming, requiring expensive equipment and skilled operators.
Lactulose (4-O-ß-galactopyranosyl-D-fructofuranose) is formed by isomerisation of lactose (4-O-ß-galactopyranosyl-D-glucopyranose) during the heat treatment of milk, and has been proposed by the International Dairy Federation (IDF)9 and by the European Commission (EC)10 as an analytical index to distinguish UHT milk from sterilised milk. The detection of lactulose is mainly based on gas chromatography,11 liquid chromatography,9 spectrophotometric methods12,13 and enzymatic methods with either spectrophotometric14,15 or amperometric detection.16,17 Our group has developed several methods for the determination of lactulose in milk samples, aimed at producing much simpler and shorter protocols that do not require expensive equipment.18–20
Immunological methods for the measurement of the extent of protein lactosylation have been developed based on the well known antigenic properties of lactose.21 Several groups have reported the production of polyclonal antibodies for lactosylated proteins21–27 using different immunogens such as lactosylated proteins and peptides, but to date no-one has been able to set up a simple ELISA procedure to distinguish among different classes of milk. All of the immunoassays proposed so far require long pre-treatment of the samples, such as protein precipitation and further purification.
The aim of this paper is to report the characterisation of monoclonal antibodies for lactosylated proteins and their use in an immunoassay [enzyme-linked immunosorbent assay (ELISA)] for the detection of lactosylated proteins in milk samples. Two commercially available classes of milk (UHT and pasteurised) treated with different heating procedures were tested in order to assess the applicability of the monoclonal antibodies. This assay does not need any pre-treatment of the milk samples other than a dilution step and seems suitable for the measurement of the extent of protein lactosylation in milk.
Experimental
Reagents and materials
All proteins, carbohydrates, chemicals for buffers and gels were obtained from Sigma (St Louis, MO, USA). Anti-mouse HRP conjugated Ab was purchased from Bio-Rad Laboratories (Richmond, CA, USA). ELISA plates were Maxisorp from Nunc (Roslklde, Denmark). Protein A for antibody purification was Protein A Sepharose 4 Fast Flow (Amersham Pharmacia Biotech, Uppsala, Sweden). Culture media for hybridoma cells was GIBCO BRL (Life Technologies, UK) and HAT medium was obtained from Flow Laboratories (USA).Skimmed milk was purchased directly in shops; all the brands tested are commercially available in Rome, Italy.
Apparatus
A Model 550 microplate reader (Bio-Rad) was used to read the absorbance on ELISA plates at 490 nm. A model 238 Uvicord S II instrument from LKB (Bromma, Sweden) at 280 nm was used for the detection of proteins during the collection of antibodies in the purification process.Protein lactosylation
Lactosylated proteins were prepared according to the procedure described by Matsuda et al.21,22 with some modifications. Ovalbumin (Ova), casein (Cas), ß-lactoglobulin (LG) and hemocyanin keyhole limpet (KLH) were lactosylated and used as standards for the antiserum characterisation and milk tests and for animal immunisation. The same procedure was used for lactosylation and the modified protein was injected into the animals as antigen for the production of antibodies. The proteins were dissolved in distilled water (2% w/v) and mixed with D-lactose (30% of the protein dry weight). The protein–sugar solutions were adjusted to pH 8.0 with dilute NaOH and freeze-dried. The dried samples were kept at 50 °C and 65% relative humidity using a saturated KI solution for 72 h, and subjected to gel filtration on a Sephadex G-50 column to eliminate any unreacted lactose. The protein concentration was determined spectrophotometrically.28 Lactosylated and native proteins were both run on sodium dodecyl sulfate polyacrylamide gel,29 as a control of the lactosylation process, by comparing sample mobilities.Immunisation procedures
Ten mice (female Balb/c) were immunised by intraperitoneal injection of lactosylated KLH (0.1 mg ml−1, 1 ml per mouse) emulsified with complete Freund’s adjuvant (CFA, Sigma). Injections were repeated several times every 4 weeks with phosphate-buffered saline (PBS)–lactosylated KLH (1 mg ml−1). Blood was collected and centrifuged and serum was stored at −20 °C until use.Production of monoclonal antibodies (mAb)
Somatic hybridisation was performed by incubating for 1 min at 37 °C 108 immune spleen cells with 107 mouse myeloma cells (NSO) in presence of 0.5 ml of 15% v/v dimethyl sulfoxide and 41% w/v polyethylene glycol (PEG). A 0.5 ml volume of 25% w/v PEG was then added. Three minutes later, fused cells were suspended slowly in 48 ml of complete culture medium (CCM) supplemented with 20% fetal bovine serum (FBS), (GIBCO) and plated on to 96-well tissue culture plates (0.2 ml per well). HAT medium (0.15 ml per well) was added on days 2 and 8, after removing 0.15 ml of spent medium. Cells from positive wells were cloned twice to 0.1 and 0.3 cells per well by limiting dilution in 96-well plates screened for the presence of antibody able to bind L-Ova or L-LG by direct ELISA (see later). Selected clones were grown in 15 ml flasks and the supernatants were used as the source of mAbs.Antibody purification
Antibodies were purified by affinity chromatography on a 10 ml protein A column (Protein A Sepharose 4 Fast Flow, 5 ml gel), the whole system consisting of the chromatographic column attached to a UV reader (λ = 280 nm) and a chart recorder. The column was subjected to sequential pre-washing with the following solutions: PBS (pH 7.4), 0.7% NaCl, 1 M NH4SO4 (pH 9), 3.5 M MgCl2 and 1 M NH4SO4 (pH 9). The hybridoma supernatant diluted in NH4SO4 (1 ml of supernatant in 9 ml of NH4SO4) was allowed to run overnight with a continuous flow system. The next day the antibodies were eluted with MgCl2 and the protein fraction was collected. Antibodies were dialysed in de-ionised water overnight and their titre was determined28 spectrophotometrically.Antibody characterisation
The binding specificity of the sugar-specific antibody for the lactosylated proteins was measured by competitive and non-competitive ELISA.30 Both polyclonal and monoclonal antibody screening were performed by coating the plates with all of the lactosylated protein standards (1 μg ml−1 in 50 mmol l−1 carbonate buffer (pH 9) overnight at 4 °C (100 μl per well)). The plate was washed three times with PBS, blocked for 1 h at 37 °C with 0.5% gelatine in PBS (200 μl per well) and then washed three times with PBS containing 0.05% Tween 20 (polyoxyethylene sorbitan monolaureate) (Sigma) (PBST).In direct ELISA, serial dilutions of serum or hybridoma cell-culture supernatant or purified antibodies (100 μl per well) were incubated at 4 °C for 2 h. The wells were then washed three times (PBST), and further incubated for 1 h at 4 °C with 0.1 ml of horseradish peroxidase-conjugated goat anti-mouse IgG (Bio-Rad). After washing (PBS), 0.1 ml per well of o-phenylenediamine in 0.1 M citrate–phosphate buffer (pH 4.5) containing 1 mM H2O2 was added (20 min at room temperature). The enzymatic reaction was stopped with 2 M HCl and the absorbance was read on an ELISA plate reader at 495 nm.
Competition curves were obtained mixing 50 μl of sample (as stated) with 50 μl of antiserum or hybridoma cell-culture supernatant or purified antibodies at the appropriate dilution in the wells and incubated for 1 h at 4 °C. The plates were then processed exactly as before. Western immunoblot31 on lactosylated and native proteins with purified antibodies was carried out to confirm antibody specificity.
Competitive ELISA in milk samples
Pasteurised and UHT milk samples were tested in competitive ELISA and the total milk protein concentration was determined spectrophotometrically.28 Serial milk dilutions from 1∶500 (∼60 μg ml−1, considering that the average protein concentration is 3.1%) to 1∶500000 (∼0.06 μg ml−1) in PBS were incubated with the appropriate mAb dilution (1 μg ml−1) and added to the plate (incubation for 1 h at 4 °C). The ELISA plates were then processed as before. The data obtained from each competition curve were plotted and fitted using SigmaPlot software (SPSS) and a regression analysis on the linear portion of the sigmoidal curves was performed. The slopes obtained for the regression analysis were used as an index of protein lactosylation.Each experiment was performed in triplicate and the mean of each value was used for curve fitting.
Lactulose determination in milk samples
Lactulose was determined according to the procedure described by Amine et al.20 using a spectrophotometric assay.Results and discussion
Protein lactosylation
Lactosylated and native proteins were subjected to 15% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) to compare the difference in mobility and to evaluate the molecular weight of lactosylated samples. As expected, lactosylated proteins had a lower mobility than the native proteins because of the higher molecular weight. The molecular weights of Ova and L-Ova were calculated to be 45000 and 48000, respectively, LG and L-LG 25000 and 30000, respectively, and Cas and L-Cas 58000 and 62000, respectively; these are comparable to the molecular weights reported by Matsuda et al.21Characterisation of the antibodies
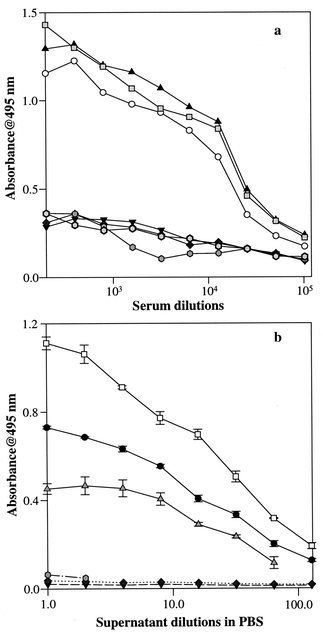 | ||
Fig. 1 (a) Polyclonal antibody selectivity for lactosylated vs. native
proteins: L-Cas (○); L-LG (![[square tinted (heavy)]](https://www.rsc.org/images/entities/char_e069.gif) );
L-Ova (▲); Cas (▼); LG (◆); Ova ( );
L-Ova (▲); Cas (▼); LG (◆); Ova ( );
no coating (♦). (b) Monoclonal antibody selectivity for lactosylated
vs. native proteins: L-Cas (●); L-LG
(□); L-Ova ( );
no coating (♦). (b) Monoclonal antibody selectivity for lactosylated
vs. native proteins: L-Cas (●); L-LG
(□); L-Ova ( ); Cas (▼); LG (♦); Ova
( ); Cas (▼); LG (♦); Ova
( ). ). | ||
As expected, mAb and pAb present different responses to lactosylated proteins; this is more evident by the competition curves reported in Fig. 2. The main difference that should be pointed out is in selectivity (Fig. 1) and sensitivity (Fig. 2; different working ranges for mAb and pAb). The positive hybridoma clone (mAb) was selected for the assay because it gives major advantages for the standardisation of the procedure. In fact, the analyst is able to operate with a reagent (mAbs) exhibiting constant characteristics in terms of selectivity, affinity and cross-reactivity with any structure related molecule. Moreover, no further immunisation (or sacrifice) is needed, nor re-optimisation of the assay conditions owing to the large variability in the immunogenic responses of different animals.
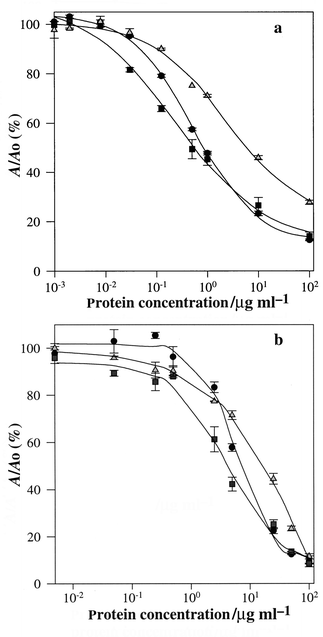 | ||
Fig. 2 (a) Polyclonal antibody competition curves with lactosylated proteins
standards: L-Ova (●); L-Cas (![[square tinted (heavy)]](https://www.rsc.org/images/entities/char_e069.gif) );
L-LG ( );
L-LG ( ). (b) Monoclonal antibody competition curves with
lactosylated proteins: L-Ova (●); L-Cas
( ). (b) Monoclonal antibody competition curves with
lactosylated proteins: L-Ova (●); L-Cas
(![[square tinted (heavy)]](https://www.rsc.org/images/entities/char_e069.gif) ); L-LG ( ); L-LG ( ). ). | ||
The affinity constants for mAbs were determined from the competition curves with lactosylated standards by the method of Friguet et al.32 [Fig. 2(b)]. The values obtained were 10−8–5 × 10−9 for L-Ova, 5 × 10−8–8 × 10−8 for L-Cas and 10−8–5 × 10−8 for L-LG.
The working range and the detection limit for L-Ova were also determined from the competition curve in Fig. 2(b). The detection limit, defined as the concentration of lactosylated standard protein equivalent to three standard deviations at Ao (no competition) was 0.015 μg l−1. The working range for L-Ova, defined as the standard protein concentration range between 90 and 10% of the maximum signal (A0)33 was 0.010–40 μg ml−1. Since mAbs had the lowest affinity for L-Ova, the latter was selected for all further experimental work. In fact, in a competitive assay format with the antigen competing for a limiting amount of mAbs, the use of a L-OVA is expected to increase the sensitivity of the assay; the majority of milk proteins is composed of casein. The same analytical behaviour was observed using a 1∶8 dilution of supernatant in PBS or 1 μg ml−1 of purified mAbs.
Cross-reactivity (CR%) was expressed as the ratio of the concentration of free L-Ova that binds to 50% of mAb used for the competition (x) to the concentration of the competitor that binds the same amount of antibody (y) (CR% = x/y).34 The results in Table 1 show that none of these molecules is able to interfere significantly with the detection of lactosylated proteins. Galactose and the galactose–ovalbumin adduct exhibited the highest cross-reactivity, suggesting that the galactose residue is probably recognised by the antibodies. Their effect is negligible considering both their CR% (15 and 10%, respectively) and the very low concentration of galactose in milk. The latter consideration applies also to lactulose. The selectivity of the mAbs versus lactose was very high. However, considering the concentration in milk samples, it was calculated that a minimum dilution of 1∶100 was required in order to have a negligible effect on the assay.
| Competitor | y/μg ml−1 | CR% |
|---|---|---|
| L-Ova | 0.88 | 100.0 |
| Ovalbumin–glucose | 174.5 | 0.5 |
| Ovalbumin–galactose | 8.64 | 10.2 |
| Ovalbumin–lactulose | 1534 | <0.1 |
| Glucose | 180.2 | 0.5 |
| Galactose | 5.9 | 15 |
| Lactose | 44 | 2 |
| Lactulose | 17.2 | 5 |
Measurement in milk samples
Preliminary experiments performed on milk samples kept at 37 °C for up to 4 d demonstrated that it was possible to follow the increase in the extent of lactosylation by a direct ELISA using polyclonal antibodies.35 A higher absorbance value was registered for the heated milk samples during a 4 d period of time versus the same samples kept at 4 °C.The purpose of this work was to produce antibodies and optimise an ELISA competitive assay able to work in milk samples with no pre-treatment of the sample other than dilution. The extent of lactosylation was evaluated by running the competitive assay for different dilutions of milk and plotting the absorbance against the total amount of proteins of the sample calculated as reported in the Experimental section.28 The total milk protein concentration range chosen for ELISA was from ∼120 to ∼0.06 μg ml−1 (corresponding to a dilution range of 1∶250 to 1∶500000). Typical competition curves for pasteurised and UHT milk are shown in Fig. 3.
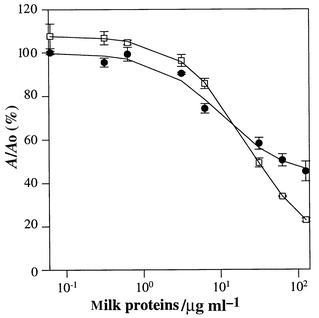 | ||
| Fig. 3 Competitive ELISA for pasteurised milk (●) and UHT milk (□) samples. | ||
Our main goal was to determine whether this immunoassay was able to distinguish between commercial classes of milk. Five pasteurised and five UHT commercial milk brands were analysed for this purpose. The increase in the severity of heat treatment in milk pasteurisation processes enhances the number of epitopes (lactosylation sites) on the milk protein. This influences the shape of the ELISA competition curves, the two main differences being the difference in the absorbance between the highest and lowest competition values and the slope of the linear portion of the sigmoidal curves. As expected, UHT milk presents a more negative slope because of the larger amount of lactosylated protein (see Fig. 3). The difference in absorbance is larger compared with fresh milk samples for the same reason, the greater amount of antigen able to bind a larger amount of mAb used in the assay.
The ELISA lactosylation index chosen to compare the results obtained for all of the samples was the slope of the competition curves between 60 and 3 μg ml−1 total protein concentration (corresponding to 1∶500 to 1∶10000 dilutions, respectively). Four different dilutions in triplicate were considered for the regression analysis and only r2 ≥ 0.990 was considered acceptable. The intra- and inter-day reproducibility of the slope were evaluated on sample No. 8 (six replicates for five days); The RSD was 3% intra-day and 8% inter-day. Data obtained for milk samples are reported in Table 2.
| Pasteurised milk sample No. | Slope | Lactulose/ mg l−1 | UHT milk sample No. | Slope | Lactulose/ mg l−1 |
|---|---|---|---|---|---|
| 1 | −0.21 | nd | 6 | −0.35 | 236 |
| 2 | −0.21 | nd | 7 | −0.43 | 321 |
| 3 | −0.19 | nd | 8 | −0.33 | 220 |
| 4 | −0.21 | nd | 9 | −0.34 | 215 |
| 5 | −0.23 | nd | 10 | −0.48 | 352 |
The lactulose content of the samples was also measured with a spectrophotometric procedure. Lactulose was not detectable in pasteurised milk samples since this method has a detection limit of 10 mg l−1.20 The results obtained for UHT milk show the same trend as for the lactosylated proteins: the higher the amount of lactulose, the more negative is the slope of the ELISA. To prove that the difference between the two classes of milk reported in Table 2 was significant, a t-test on the sample means was performed. The probability that this difference is relevant was > 99% at the 95% confidence level, meaning that this difference is statistically relevant.
Conclusions
Monoclonal antibodies for lactosylated proteins were produced, characterised and used in a competitive assay format. The data obtained indicate that the ELISA developed is applicable to diluted milk samples and is able to distinguish between milk samples that have undergone different heat treatments (UHT and pasteurised milk).Acknowledgement
This work was supported by the EU, project EU-FAIR CT 96-1095.References
- M. A. J. S. van Boekel, Food Chem., 1999, 62, 403 CrossRef.
- J. O’Brien and P. A. Morrisey, Crit. Rev. Food Sci. Nutr., 1989, 28, 5 Search PubMed.
- J. Mauron, Prog. Food Nutr. Sci., 1981, 5, 5 Search PubMed.
- P. A. Finot, R. Deutsch and E. Bujard, Prog. Food Nutr. Sci., 1981, 5, 345 Search PubMed.
- P. Resmini, L. Pellegrino and G. Battelli, Ital. J. Food Sci., 1990, 3, 173.
- J. Hartkporf and H. F. Ebersdobler, J. Chromatogr., 1993, 635, 151 CrossRef.
- A. Tirelli and L. Pellegrino, Ital. J. Food Sci., 1995, 7, 379 CAS.
- R. Acquistucci, G. Panfili and E. Marconi, J. Agric. Food Chem., 1996, 44, 3855 CrossRef CAS.
- IDF, Standard 147, International Dairy Federation, Brussels, 1991..
- EC Commision, Dairy Chemists’ Group-Doc VI/5276, European Commission, Brussels, 1992..
- A. Olano, M. M. Calvo and V. Corzo, Food Chem., 1989, 31, 259 CrossRef CAS.
- A. K. Adhikari, D. Sahai and O. N. Mathur, Lait, 1991, 71, 555 Search PubMed.
- F. W. Parish, K. Hicks and L. Donner, J. Dairy Sci., 1980, 63, 1809 Search PubMed.
- H. Geier and H. Klostermeyer, Z. Lebensm.-Unters. Forsch., 1980, 171, 443 Search PubMed.
- G. R. Andrews, J. Soc. Dairy Technol., 1984, 37, 92 Search PubMed.
- M. Mayer, M. Genrich, W. Kunnecke and U. Bilitewski, Anal. Chim. Acta, 1996, 324, 37 CrossRef CAS.
- Y. Sekine and E. A. H. Hall, Biosens. Bioelectron., 1998, 13, 995 CrossRef CAS.
- D. Moscone, R. A. Bernardo, E. Marconi, A. Amine and G. Palleschi, Analyst, 1999, 124, 325 RSC.
- A. Amine, D. Moscone and G. Palleschi, Anal. Lett., 2000, 33(1), 125.
- A. Amine, D. Moscone, R. A Bernardo, E. Marconi and G. Palleschi, Anal. Chim. Acta, 2000, 406, 217 CrossRef CAS.
- T. Matsuda, Y. Kato, K. Watanabe and R. Nakamura, J. Food Sci., 1985, 50, 618 Search PubMed.
- T. Matsuda, Y. Kato, K. Watanabe and R. Nakamura, J. Agric. Food Chem., 1985, 33, 1193 CrossRef CAS.
- T. Matsuda, Y. Kato, K. Watanabe and R. Nakamura, Mol. Immunol., 1987, 24, 421 CrossRef CAS.
- T. Matsuda, Y. Kato and R. Nakamura, J. Agric. Food Sci., 1991, 39, 1201 CrossRef CAS.
- T. Matsuda, H. Ishiguro, I. Ohkubo and R. Nakamura, J. Biochem., 1992, 111, 383 Search PubMed.
- V. Fogliano, S. M. Monti, A. Ritieni, C. Marchisano, G. Peluso and G. Randazzo, Food Chem., 1997, 58, 53 CrossRef CAS.
- R. Pizzano, M. A. Nicolai, R. Siciliano and F. Addeo, J. Agric. Food Sci., 1998, 46, 5373 CrossRef CAS.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biochem., 1951, 193, 265 Search PubMed.
- U. K. Laemmli, Nature (London), 1979, 227, 680.
- E. Engval and P. Perhann, Immunochemistry, 1971, 8, 871 Search PubMed.
- H. Towbin, T. Staehelin and J. Gordon, Proc. Natl. Acad. Sci. USA, 1974, 76, 4350.
- B. Friguet, A. F. Chaffotte, L. Djavadi-Ohaniance and M. J. Goldberg, J. Immunol. Methods, 1985, 77, 305 CrossRef CAS.
- G. Giraudi, I. Rosso, C. Baggiani, C. Giovannoli, A. Vanni and G. Grassi, Anal. Chim. Acta, 1999, 392, 85, and references cited therein. Search PubMed.
- M. Pallini, D. Compagnone, S. Marini and G. Palleschi, in Proceedings of the 4th Italian Conference on Sensors and Microsystems, ed. C. Di Natale, A D’Amico and F. Davide, World Scientific, Singapore, 2000, pp. 63–68. Search PubMed.
- G. E. Abraham, J. Clin. Endocrinol. Metab., 1696, 29, 866 Search PubMed.
| This journal is © The Royal Society of Chemistry 2001 |
