Electrochemical detection of sequence-specific DNA using a DNA probe labeled with aminoferrocene and chitosan modified electrode immobilized with ssDNA
Chun Xu, Hong Cai, Pingang He and Yuzhi Fang*
Department of Chemistry, East China Normal University, Shanghai, 200062, China.. E-mail: yuzhi@online.sh.cn;; Fax: +86 21 62451921
First published on 18th December 2000
Abstract
The electrochemical detection of sequence-specific DNA using a DNA probe labeled with aminoferrocene (AFC) is reported. Sample ssDNA was immobilized on a chitosan modified glassy carbon electrode. A sequence-known DNA with 256 bp [obtained by polymerase chain reaction (PCR)] was successfully labeled with the electro-active reagent AFC by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide for the first time. This DNA probe labeled with AFC was applied to hybridize with a sequence-unknown DNA sample. Only the complementary sequence (cDNA) could form a double-stranded DNA (dsDNA) with the DNA probe labeled with AFC. The anodic peak currents (ipa) of the AFC bound to the dsDNA by differential pulse voltammetry were used for the determination of cDNA. The ipa of AFC was linearly related to the concentration of cDNA sequence between 1.0 × 10−8 and 6.0 × 10−6 mol L−1. The detection limit was 2.0 × 10−9 mol L−1 using 3σ (where σ is the standard deviation of blank solution, n = 11). The probe showed high sensitivity and selectivity.
Introduction
DNA hybridization biosensors have been a very popular topic during the past several years and they hold an enormous promise for the clinical diagnosis of inherited diseases and the rapid detection of infectious microorganisms. Various techniques of biotin,1 fluorescent dye,2 chemiluminophore,3 surface plasmon resonance sensor4 and electrochemical DNA probe,5–10 aiming at detecting the base pair hybridization between a labeled probe and a target DNA, have been developed. Since electrochemical techniques can offer the advantages of being cheap, sensitive and rapid, many electrochemical DNA biosensors for sequence-specific DNA detection have been reported.11–26 For example, Wang’s group13 employed DNA probes for the detection of point mutations in the p53 gene. The same group developed an electrochemical biosensor for detecting the Mycobacterium tuberculosis DNA in connection to a Co(phen)3 indicator.14 Hashimoto et al.15,16 reported a DNA electrochemical sensor based on an intercalator as a hybridization indicator. Millan and Mikkelsen17,18 described a sequence-selective biosensor using a DNA-modified glassy carbon electrode and Co(pby)33+ as electroactive hybridization indicator. Ihara et al.19 employed ferrocene-mediated oligonucleotides for a sandwich-based electrochemical detection of DNA hybridization. Takenaka’s team20 reported on a naphthalene–ferrocene redox indicator with a remarkable discrimination between the probe and duplex. Our group has also reported several electrochemical DNA biosensors in specific DNA sequence detection21–24 and damage and protection of DNA.25,26In this paper, aminoferrocene (AFC) was labeled with a sequence known DNA with 256 bp (obtained by the polymerase chain reaction (PCR), marked as PCR DNA) by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) for the sequence-specific DNA detection. The use of AFC was one of the novelties of this work. Its advantages lie in that first, it avoids the use of toxic anticarcinogens, such as daunomycin25 and ethidium bromide21etc. Secondly, the adsorption of the AFC labeled probe on the electrode is weak, so the interference caused by the common intercalators which are used as hybridization indicator15–18 could be eliminated. Thirdly, the labelling procedure of this method is simpler than that of the previous published work;23 AFC was directly labeled on the 5′ end of PCR DNA without using ethylenediamine as a linking reagent. The other novelty was that the chitosan oligomer film was for the first time used as an active coating for the immobilization of ssDNA at a glassy carbon electrode. Chitosan oligomer is a kind of β-1,4-linked glucosamine oligomer;27 it is a natural cationic polymer and can form a stable complex with the polyanionic phosphodiester backbones of DNA, either native or denatured.28 The main advantages of using chitosan were that chitosan could form a tight complex with DNA which made the immobilization very stable. Compared with the DNA immobilization methods using self-assembly monolayer (SAM) and biotin, the use of chitosan for DNA immobilization did not need the mercapto–DNA11 and biotin–DNA,1 which could greatly reduce the detection cost. Further study of the electrochemical characterization of the chitosan modified electrode for ssDNA immobilization is proceeding.
Experimental
Chemicals and solutions
The sequence-known DNA with 256 bp (obtained by the polymerase chain reaction (PCR), A260/A280 = 1.96, marked as PCR DNA) and DNA plasmid pNC3 (about 4000 bp, containing a sequence complementary to the PCR DNA, A260/A280 = 1.63; no further purification) were obtained from the Molecular Biology Laboratory, Biology Department, East China Normal University (Shanghai, China). Calf-thymus DNA was purchased from Baitai Biochemical Technology Company (Beijing, China), Sperm DNA and yeast RNA was purchased from Shanghai Institute of Biochemistry (Chinese Academy of Sciences, Shanghai, China), λDNA and pBR322 DNA were purchased from Huamei Biotechnology Company (Shanghai, China). The DNA concentration was determined spectrophotometrically using the known molar absorption coefficient 6600 (mol L−1)−1 cm−1 at 260 nm (per P or nucleotide unit).29 Denatured single-stranded DNA (ssDNA) was produced by heating native double-stranded DNA in a boiling water bath for about 5 min followed by rapid cooling in an ice bath. Chitosan oligomer (1.0% solution in 1.0% acetic acid) was purchased from Aldrich (USA). Aminoferrocene was obtained from Organic Laboratory of Chemistry Department, East China Normal University (Shanghai, China). 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and hydroxymethylaminomethane (TRIS) were purchased from Sigma (USA), N-methylimidazole and sodium dodecylsulfate (SDS) were obtained from Guangyao Chemical Reagent Company (Jiangsu, China). The following buffers were used: 2 × SSC buffer: 0.3 mol L−1 NaCl + 0.03 mol L−1 sodium citrate (pH 7.0) and TE buffer: 10 mmol L−1 TRIS–HCl + 1.0 mmol L−1 EDTA (pH 8.0).Other reagents were commercially available and were all of analytical reagent grade. Solutions were prepared with distilled water.
Instrumentation
A CHI 630 Electrochemical Analyzer (CHI Instruments Inc. USA), a JB-1 stirring machine (Branson, Shanghai, China) and a TDL-16B centrifuge (Anting Science Instrument Inc., Shanghai, China) were used.The three-electrode system consisted of a working electrode made of glassy carbon (3 mm id), an Ag/AgCl (KCl, 3.0 mol L−1) reference electrode and a counter electrode made of platinum. All measurements were carried out in a 10 mL cell.
Procedure
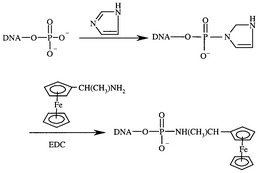 | ||
| Fig. 1 Synthesis of DNA probe labeled with aminoferrocene. | ||
The chitosan modified electrode was immersed in a 1.0 mL TE solution containing 6.06 × 10−6 mol L−1 denatured sample DNA solution (ssDNA). The solution was stirred at room temperature (25 ± 0.5 °C) for 120 min. Thus the ssDNA was immobilized on the chitosan-modified electrode (the immobilization schematic diagram of ssDNA on the chitosan modified glassy carbon electrode is shown in Fig. 2). Then, the electrode was washed with 0.1% (m/m) SDS phosphate buffer (pH 7.0) three times and immersed in a 0.01 mol L−1 TE buffer (pH 8.0) prior to use.
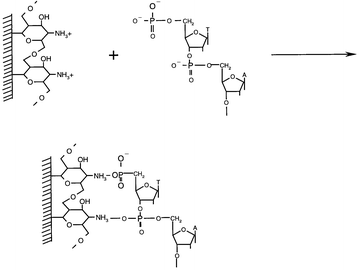 | ||
| Fig. 2 Immobilization schematic diagram of ssDNA on chitosan-modified glassy carbon electrode. | ||
Results and discussion
Cyclic voltammograms of the DNA probe labeled with AFC in solution
Cyclic voltammograms of the DNA probe labeled with AFC in a 0.01 mol L−1 TE solution using bare glassy carbon as working electrode was recorded at a scan rate of 100 mV s−1 and scan range from −0.20 to +0.60 V (vs. Ag/AgCl). A pair of redox peaks was obtained at +0.29 V and +0.20 V (vs. Ag/AgCl), respectively, which were the redox peaks of the aminoferrocene. With increasing concentration of the DNA probe labeled with AFC, the anodic peak current increased obviously (Fig. 3a, b and c). It indicated that the DNA probe labeled with AFC had a good electrochemical response at the glassy carbon electrode.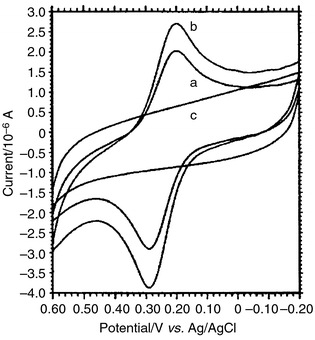 | ||
| Fig. 3 Cyclic voltammograms of the DNA probe labeled with aminoferrocene in 0.01 mol L−1 TE solution, scan range −0.20–+0.60 V (vs. Ag/AgCl), scan rate 100 mV s−1. The concentration of the aminoferrocene labeled DNA probe was (a) 5.0 × 10−7 mol L−1 and (b) 1.0 × 10−6 mol L−1. (c) The blank TE solution without the DNA probe labeled with aminoferrocene. | ||
Sequence-specific DNA diagnosis
The principle of DNA probes labeled with electroactive reagents for sequence-specific DNA detection is based on the fact that the sample ssDNA is immobilized at an electrode surface before it is hybridized with the sequence-known DNA probe, which is already labeled with an electroactive reagent, i.e. AFC. Only the complementary sample DNA can form a double stranded DNA with the electroactive reagent labeled probe, and be diagnosed using electrochemical techniques.The proposed DNA probe labeled with AFC was applied to hybridize with sample DNA sequences (all at a concentration of 2.5 × 10−7 mol L−1) immobilized on the surface of the chitosan modified glassy carbon electrode. Differential pulse voltammograms were recorded using the dsDNA/AFC electrode as the working electrode in a blank TE solution. The anodic wave derived from AFC bound to the dsDNA on the electrode surface appeared only at the denatured plasmid DNA electrode by DPV detection. As shown in Fig. 4a, the anodic peak current of AFC was obtained at +0.25 V (vs. Ag/AgCl) and its value was 3.82 μA. Other noncomplementary sequence electrodes, namely denatured calf-thymus DNA, denatured sperm DNA, denatured λDNA, denatured pBR322 DNA and yeast RNA, showed no electrochemical responses ranging from 0.00 to +0.80 V (vs. Ag/AgCl) in the case of DPV detection (shown in Fig. 4b, the denatured calf-thymus DNA electrode was used as to represent these noncomplementary DNA electrodes). These data indicated that the DNA probe labeled with AFC could distinguish between the complementary DNA sequence and the noncomplementary DNA sequences and it showed high selectivity.
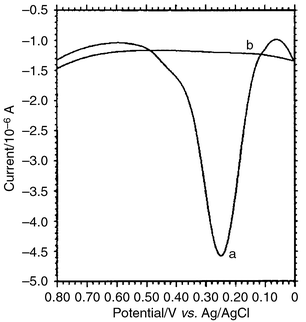 | ||
| Fig. 4 Differential pulse voltammograms of dsDNA/AFC electrode in a 0.01 mol L−1 blank TE solution, scan range, 0.00–+0.80 V (vs. Ag/AgCl), scan rate, 100 mV s−1. Chitosan amount (1.0% solution in 1.0% acetic acid), 2.0 μL; immobilization time, 120 min; hybridization time, 60 min. The DNA probe labeled with AFC hybridizes with (a) denatured plasmid DNA electrode, (b) noncomplementary DNA electrode. | ||
Condition optimization
Factors affecting the immobilization of denatured sample DNA and hybridization were optimized aiming at maximizing the sensitivity and shortening the detection time. As shown in Fig. 5, using the AFC labeled DNA as an indicator, the peak current of chitosan-modified electrode increased rapidly with increase of the applied amount of chitosan at the electrode. When chitosan was over 2.0 μL, the increase of the peak current leveled off (Fig. 5A), so the appropriate amount of chitosan was 2.0 μL. The influence of immobilization time was examined from 10 to 150 min. The current of AFC increased gradually with the increase of the immobilization period up to 120 min, and then it leveled off (Fig. 5B). This showed that the surface was saturated with denatured sample DNA. Fig. 5C demonstrates the effect of the pH in the hybridization solution. As can be seen, the pH value has no pronounced effect on the hybridization (in the range of pH 5–8). The effect of the hybridization time is displayed in Fig. 5D. As the hybridization time increased, the current of AFC increased rapidly at beginning (up to 60 min) and then leveled off slightly. Based on the data of Fig. 5, as the best compromise between sensitivity and speed, most work employed a 120 min immobilization time, a 60 min hybridization time and a 2 × SSC buffer (pH 7.0).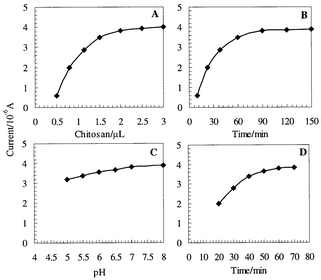 | ||
| Fig. 5 Effect of (A) chitosan amount, (B) immobilization time, (C) pH value and (D) hybridization time in a 0.01 mol L−1 blank TE solution. The DNA probe labeled with AFC was used as an indicator. Other conditions were the same as in Fig. 4. Each value was the mean of at least seven replicate measurements. | ||
Linearity and detection limits
The linearity of the denatured plasmid DNA was investigated by varying its concentration over the range of 1.0 × 10−8–6.0 × 10−6 mol L−1 (TE buffer, 120 min immobilization and 60 min hybridization). The well-defined DPV peak of AFC increased linearly with increasing concentration of the denatured plasmid DNA. The regression equation was Y = 1.2173 × 107X + 1.080 (X was the concentration of denatured plasmid DNA from 1.0 × 10−8 to 6.0 × 10−6 mol L−1, Y was the peak current; the unit was μA) and regression coefficient (r2) of the linear curve was 0.9971. The detection limit based on 3σ (where σ is the standard deviation of a blank solution, n = 11) was 2.0 × 10−9 mol L−1.Reproducibility and stability
The reproducibility was estimated by making repetitive hybridizations with denatured plasmid DNA according to the specified procedure. The relative standard deviation (RSD) based on 11 measurements for the denatured plasmid DNA was 5.3%. Only 4.2% deterioration of anodic peak currents was found during three months storage of the AFC labeled probe in a refrigerator.Conclusions
In this paper, a DNA probe labeled with aminoferrocene (AFC) was synthesized using a carbodiimide (EDC) for the first time. Meanwhile, the sample ssDNA was successfully immobilized onto a chitosan-modified glassy carbon electrode. The proposed DNA probe labeled with AFC was successfully used for the sequence-specific DNA detection. Only the complementary sequence (cDNA) could form a double-stranded DNA (dsDNA) with the DNA probe labeled with AFC. The probe showed high sensitivity and selectivity. Our future work will be focused on the further study of the electrochemical characterization of chitosan-modified electrode for ssDNA immobilization and the applications of the electrochemical DNA probe.Acknowledgements
We thank the National Nature Science Foundation of China (NSFC) which financially supports this work (No. 29875008).References
- J. Olejnik, E. Krzymanska-Olejnik and K. J. Rothschild, Nucleic Acids Res, 1998, 26, 3572 CrossRef CAS.
- T. Livache, B. Fouque, A. Roget, J. Marchand, G. Bidan, R. Teoule and G. Mathis, Anal. Biochem, 1998, 255, 188 CrossRef CAS.
- D. K. Xu, L. R. Ma, Y. Q. Liu and Z. H. Jing, Analyst, 1999, 124, 533 RSC.
- G. Marrazza, I. Chiane and M. Mascini, Biosens. Bioelectron, 1999, 14, 43 CrossRef CAS.
- E. Palecek, M. Fojta, M. Tomschik and J. Wang, Biosens. Bioelectron, 1998, 13, 621 CrossRef CAS.
- M. Fojta, V. Stankova, E. Palecek, P. Koscielniak and J. Mitas, Talanta, 1998, 46, 115 CrossRef CAS.
- S. O. Kelley, E. M. Boon, J. K. Barton, N. M. Jackson and M. G. Hill, Nucleic Acids Res, 1999, 27, 4830 CrossRef CAS.
- T. Ihara, Y. Maruo, S. Takenaka and M. Takagi, Nucleic Acids Res, 1996, 24, 4273 CrossRef CAS.
- M. T. Carter, M. Rodriguez and A. J. Bard, J. Am. Chem. Soc, 1989, 111, 8902.
- M. Rodriguez and M. T. Carter, Anal. Chem, 1990, 62, 2658 CrossRef CAS.
- S. Takenaka, K. Yakenaka, M. Takagi, Y. Uto and H. Kondo, Anal. Chem, 2000, 72, 1334 CrossRef CAS.
- K. Yamashita, S. Takenaka and M. Takagi, Nucleic Acids Symp. Ser, 1999, 42, 185 Search PubMed.
- J. Wang, G. Rivas, X. Cai, M. Chicharro, C. Parrado, N. Dontha, A. Begleiter, M. Mowat, E. Palecek and P. Nielsen, Anal. Chim. Acta, 1997, 344, 111 CrossRef CAS.
- J. Wang, G. Rivas, X. Cai, N. Dontha, H. Shiraishi, D. Luo and F. Valera, Anal. Chim. Acta, 1997, 337, 41 CrossRef CAS.
- K. Hashimoto, K. Miwa and Y. Ishimori, Superamol. Chem, 1993, 2, 265 Search PubMed.
- K. Hashimoto, K. Itoh and Y. Ishimori, Anal. Chim. Acta, 1994, 286, 219 CrossRef CAS.
- K. M. Millan and S. R. Mikkelsen, Anal. Chem, 1993, 65, 2317 CrossRef CAS.
- K. M. Millan, A. Saraullo and S. R. Mikkelsen, Anal. Chem, 1994, 66, 2943 CrossRef CAS.
- T. Ihara, M. Nakama, M. Nakano and M. Maeda, Chem. Commun, 1997, 1609 RSC.
- S. Takenaka, Y. Uto, H. Saita, M. Yokayama, H. Kondo and D. Wilson, Chem. Commun, 1998, 1111 RSC.
- S. H. Liu, J. N. Ye, P. G. He and Y. Z. Fang, Anal. Chim. Acta, 1996, 335, 239 CrossRef CAS.
- X. Y. Sun, P. G. He, S. H. Liu, J. N. Ye and Y. Z. Fang, Talanta, 1998, 47, 487 CrossRef CAS.
- C. Xu, P. G. He and Y. Z. Fang, Anal. Chim. Acta, 2000, 411, 31 CrossRef CAS.
- C. Xu, H. Cai, P. G. He and Y. Z. Fang, Chem. J. Chin. Univ, 2000, 21, 1187 Search PubMed.
- X. Y. Sun, C. Xu, S. H. Liu, P. G. He and Y. Z. Fang, Chem. J. Chin. Univ, 1998, 19, 1393 Search PubMed.
- C. Xu, H. Cai, P. G. He and Y. Z. Fang, Fresenius’ J. Anal. Chem, 2000, 367, 593 CrossRef CAS.
- Y. H. Yu and B. L. He, Polym. Bull, 1997, 12, 232.
- H. Hayatsu, Y. Tanaka and K. Negishi, Nucleic Acids Symp. Ser, 1997, 37, 139 Search PubMed.
- T. Motomura and Y. Aoyama, Bull. Chem. Soc. Jpn, 1992, 65, 1755 CAS.
| This journal is © The Royal Society of Chemistry 2001 |
