New reactivity patterns in the Diels–Alder reactivity of nitrobenzofuroxans![[hair space]](https://www.rsc.org/images/entities/h2_char_200a.gif) †
†
Patricia Sepulcria, Régis Goumont*a, Jean Claude Hallé*a, Didier Rioua and François Terrier*a
aLaboratoire SIRCOB, ESA CNRS 8086, Bâtiment Lavoisier, Université de Versailles, 45, avenue des Etats-Unis, 78035, Versailles Cedex, France. E-mail: terrier@chimie.uvsq.fr, hallé@chimie.uvsq.fr; goumont@chimie.uvsq.fr
bInstitut Lavoisier, UMR CNRS 8637, Université de Versailles, 45, avenue des Etats-Unis, 78035, Versailles Cedex, France
First published on UnassignedUnassigned14th January 2000
Abstract
The reaction of cyclopentadiene with 4-nitro-6-trifluoromethylsulfonylbenzofuroxan 5 in dichloromethane or chloroform proceeds stereoselectively at 0 °C to afford a single compound 6, which is shown to result from an inverse electron-demand Diels–Alder condensation involving the carbocyclic ring of 5 as the diene contributor. However, the adduct 6, whose X-ray structure could be obtained, is not the thermodynamically stable product of the interaction. Keeping a solution of 6 at room temperature results after a few days in the isolation of a new adduct 7a which arises from a regioselective and stereoselective normal electron-demand Diels–Alder condensation involving the C(4)C(5) double bond of 5 as the dienophile contributor. The carbodienic behaviour of 5 as well as the preferred dienophilic reactivity of the C(4)C(5) rather than of the C(6)C(7) double bond, represent two new reactivity patterns in the chemistry of the nitrobenzofuroxans.
We and other research groups have long been engaged in the study of the reactivity of nitrobenzofuroxans that show an extremely high susceptibility to covalent nucleophilic addition or substitution processes.1–4 These studies have led to numerous synthetic, biological and analytical applications, most of them being centered on the use of the readily available 4,6-dinitro derivative, commonly referred to as DNBF (see structure in Scheme 1).1,5–10
 | ||
| Scheme 1 | ||
It has been argued that the low aromatic character of the benzofuroxan system is one of the major factors responsible for the exceptional or super-electrophilic reactivity of DNBF and nitrobenzofuroxans in general.1,9 Interestingly, Kresze and Bathelt reported in 1973 the very slow formation of the diadducts 1a and 1b upon treatment of DNBF with butadiene and 2,3-dimethylbutadiene, respectively.11 Although the formation of these compounds was reasonably accounted for in terms of normal electron demand Diels–Alder (NEDDA)-type processes, this promising discovery did not lead to further investigations and neither the stereochemistry nor the mechanistic sequence leading to 1a and 1b were elucidated.
Recent studies in our laboratory have revealed that DNBF is in fact a very versatile Diels–Alder reagent, being capable of acting either as a dienophile or as a heterodiene, depending upon the experimental conditions and the reaction partners employed.12,13Scheme 1 gives an example of this potentially ambident behaviour with the finding that the reaction of DNBF with an excess of cyclopentadiene affords initially a mixture of the normal (NEDDA) and inverse (IEDDA) electron-demand Diels–Alder adducts, 2 and 3, respectively.12c Interestingly, in this system, the highly functionalized stereoselective diadduct 4 is eventually obtained in high yield, implying a greater dienophilic reactivity of the remaining nitroolefinic moiety of the IEDDA adduct 3 than of the NEDDA adduct 2.12c
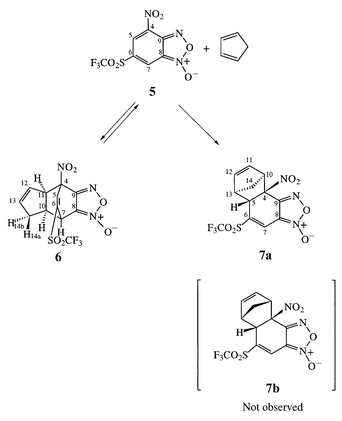
The potential importance of the Diels–Alder behaviour of DNBF for access to new heterocyclic structures prompted us to examine how changes in the nature and/or position of the substituents of the carbocyclic ring may modulate the Diels–Alder reactivity of nitrobenzofuroxan derivatives.14,15 In particular, one could anticipate that the replacement of the 6-NO2 group of DNBF by an activating substituent devoid of appreciable resonance effects might favor the dienophilic behaviour of the C(6)C(7) double bond, or induce a preferred reactivity, either dienophilic or heterodienic, at the C(4)C(5) double bond, thus opening the route to other reactivity patterns. In this regard, the SO2CF3 group appeared to be a good substituent candidate because its activating effect derives essentially from a polar effect while being of the same order as that of a NO2 group.16–21 In this work, we therefore report on the reaction of 4-nitro-6-trifluoromethylsulfonylbenzofuroxan 5 with cyclopentadiene in dichloromethane or chloroform; as will be seen, this reaction affords a thermodynamically stable cycloadduct 7a which is in fact the result of 5 acting as a dienophile through its C(4)C(5) double bond. A most noteworthy feature, however, is that the formation of 7a is preceded by that of a structurally unique monoadduct. X-Ray evidence is presented that this species arises from an inverse electron demand cyclization process in which the carbocyclic ring of 5 acts as the diene component. This behaviour, together with some other typical features, further emphasizes the multifaceted reactivity of nitrobenzofuroxans.
Results and discussion
Treatment of 5 with a large excess of cyclopentadiene (10 equiv.) in chloroform or in dichloromethane at 0 °C for five days furnished a pale yellow solid in good yield whose structure was determined by X-ray crystallography. The ORTEP view of Fig. 1 shows that this product is a cycloadduct which can be formulated as the diastereomer 6 in its racemic form (only one enantiomer is shown in Scheme 2). The stereochemistry of 6 in the crystal fully agrees with the structural information obtained from a detailed NMR analysis of the 1H and 13C NMR spectra, recorded in CDCl3 solution at 0 °C via COSY as well as NOE and J-modulation experiments (Tables 1 and 2). In particular, 2-D NOE experiments confirmed that the H(7), H(10), H(11) and H(14b) protons are in close space proximity with the three latter in a trans position to the C(7)C(6)C(5)C(4) bridge bearing the SO2CF3 substituent, in full accord with the respective distances obtained by X-ray crystallography: dH(7)–H(10) = 2.45, dH(10)–H(11) = 2.21, dH(10)-H(14b) = 2.22 Å. That the strongly electron-withdrawing SO2CF3 is bonded at the sp2 carbon C(6) is consistent with the observed fluorine resonance (δF = −77.03 ppm).22| δH | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adducts | H(5) | H(7) | H(10) | H(11) | H(12) | H(13) | H(14) | δF | Coupling constants/Hz |
| a Ref. 24. b Ref. 12(c). | |||||||||
| 6 | 8.27 | 4.58 | 3.33 | 4.27 | 5.52 | 6.05 | 2.82(a) | −77.03 | 2J14a/14b 18.7; 3J11/10 8.5; 3J12/11 2.2; 3J12/13 5.8; 3J13/14a 1.9; |
| 2.40(b) | 3J14a/10 10.0; 4J5/7 1.0 | ||||||||
| 7a | 3.58 | 7.76 | 4.15 | 6.37 | 6.74 | 3.71 | 1.84(a) | −76.50 | 2J14a/14b 10.5; 3J11/10 2.8; 3J12/11 5.7; 3J12/13 3.3 |
| 1.39(b) | |||||||||
8![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a | 3.50 | 7.06 | 4.12 | 6.34 | 6.67 | 3.45 | 1.80(a) | −65.13 | 2J14a/14b 10.3; 3J11/10 2.6; 3J12/11 5.6; 3J12/13 3.3; 4J7/F 1.3 |
| 1.41(b) | |||||||||
4![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b | 3.49 | 4.06 | 4.06 | 6.38 | 6.69 | 3.49 | 1.80(a) | — | 2J14a/14b 10.2; 3J11/10 2.8; 3J12/11 5.7; 3J12/13 3.3 |
| 1.18(b) | |||||||||
| δC | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adducts | C(4) | C(5) | C(6) | C(7) | C(8) | C(9) | C(10) | C(11) | C(12) | C(13) | C(14) | CF3 | Coupling constants/Hz | |
| a In Me2SO-d6, ref. 24. b In Me2SO-d6, ref. 12(c). | ||||||||||||||
| 6 | 87.87 | 149.35 | 137.30 | 36.77 | 110.17 | 155.16 | 42.35 | 59.15 | 124.07 | 139.63 | 36.77 | 119.34 | 1JCF3 326.1 | |
| 7a | 91.75 | 50.07 | 137.29 | 126.74 | 108.96 | 150.15 | 54.96 | 134.46 | 141.60 | 51.63 | 45.55 | 119.62 | 1JCF3 327.5 | |
8![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a | 91.59 | 48.23 | 133.98 | 113.45 | 109.11 | 150.56 | 55.03 | 134.51 | 140.98 | 49.88 | 45.66 | 122.30 | 1JCF3 274.3 | |
4![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b | 91.27 | 47.54 | 121.82 | 32.17 | 111.35 | 152.69 | 54.56 | 134.27 | 141.90 | 46.11 | 46.24 | — | ||
 | ||
| Fig. 1 ORTEP view of the adduct 6. | ||
 | ||
| Scheme 2 | ||
Despite its remarkable stability in the solid state, the adduct 6 is not the thermodynamically stable product of the reaction of 5 with cyclopentadiene. Thus major changes in the 1H and 13C spectra occurred when the temperature of a CDCl3 solution of 6 was slowly raised from 0 °C to room temperature. This first resulted in an almost complete disappearance of the signals due to 6 with a concomitant reappearance of the signals due to the starting materials. Thereafter, complex new NMR patterns slowly developed which, after a few days, clarified to a set of signals ascribable to 7a or 7b as the thermodynamically more stable product of the overall interaction. A detailed analysis of the NMR spectra recorded at the completion of the interconversion process leaves no doubt as to the identity of the product as one of the two diastereomeric NEDDA-type cycloadducts 7a and 7b. Among other diagnostic features for this structural assignment was the observation of a quaternary sp3 carbon bonded to a NO2 group (δC(4) = 91.75 ppm) as well as a 19F resonance typical for a SO2CF3 group bonded to a sp2 carbon (δF = −76.50 ppm).22,23 Despite our failure to achieve successful NOE experiments, available data support the stereoselective formation of 7a rather than of 7b. As can be seen in Tables 1 and 2, the 1H and 13C resonances pertaining to the cyclopentenyl moiety of 7a compare remarkably well with those for the same moiety in the previously isolated adducts 4 and 8, suggesting an identical configuration of this moiety in these compounds.12c,24
Thus, we have discovered at this stage, that the reaction of 5 with cyclopentadiene proceeds through a unique reactivity pattern in the chemistry of nitrobenzofuroxans. First, the initial formation of the adduct 6 under kinetic control can be reasonably visualized as arising from an inverse electron demand Diels–Alder condensation in which the carbocyclic ring of 5 acts as diene contributor, a situation which contrasts markedly with the preferred heterodienic behaviour observed in the DNBF system.12c In the DNBF molecule, the available theoretical and experimental evidence is that the conjugation between the 6-NO2 and the C(6)C(7) double bond gives rise to a heterodienyl fragment whose reactivity overcomes not only that of the C(4)C(5)C(6)C(7) carbodienic one but also that of the heterodienic counterpart involving the C(4)C(5) double bond.12,13 Interestingly, the fact that the powerful electron-withdrawing effect of the SO2CF3 group derives essentially from a polar effect rules out heterodienic behaviour of the fragment consisting of this group and the C(6)C(7) double bond in 5.20 In this instance the initial formation of 6 indicates that the carbodienic reactivity of this compound is now preferred, at least kinetically, relative to that of the heterodienic O(4)N(4)C(4)C(5) fragment.
A second noteworthy feature of the present work is that the thermodynamically more stable adduct 7a of the interaction results from a preferred dienophilic reactivity at the C(4)C(5) double bond rather than at the C(6)C(7) double bond of 5. In the DNBF systems, all known monocondensations leading to NEDDA-type adducts have involved the C(6)C(7) double bond as the dienophilic partner.11,12
Experimental
Materials
4-Nitro-6-trifluoromethylsulfonylbenzofuroxan 5 (mp 176 °C, lit.25 181 °C) was prepared from the thermal decomposition of the corresponding substituted phenyl azide in toluene.25,26 Cyclopentadiene obtained from the heating of bicyclopentadiene was used without further purification.Preparation of 6. General procedure
Excess cyclopentadiene (5 mL, >10 equiv.) was added to a solution of 1 g of 5 in CH2Cl2 or CHCl3 (5 mL) at 0 °C. The solution turned rapidly to orange and the reaction mixture was then stirred for 5 days. Addition of pentane resulted in the formation of a product which was collected by filtration and dried under vacuum. The cycloadduct was obtained as a pale yellow solid. A single crystal was obtained by recrystallization from a CHCl3–pentane mixture.Preparation of 7a
Warming the reaction mixture obtained at 0 °C to room temperature and stirring for a few days resulted in an essentially quantitative formation of the adduct 7a as pale yellow crystals which were recrystallized from a CHCl3–pentane mixture.Measurements
1H NMR, 13C NMR and 19F NMR spectra were recorded on a Bruker AC300 instrument with tetramethylsilane (TMS) as internal standard for 1H NMR, and 13C NMR and CFCl3 for 19F NMR operating, at 300, 75.5 and 282.4 MHz, respectively. Chemical shifts are reported in parts per million (ppm) and coupling constants J in Hertz (Hz). Chemical ionization mass spectra (CI) and electronic impact masss spectra (EI, 70 eV) were obtained using a HEWLETT PACKARD 5989B and a NERMAG R10-10C, respectively. IR spectra were recorded on a NICOLET 400D spectrometer.Structure determination
The X-ray structure determination of the adduct 6 has been carried out with a Siemens SMART three circle diffractometer equipped with a bidimensional CCD detector. All these data obtained, together with the various parameters of the experiments, are reported in Tables 3–5. These data were corrected for absorption effect by the SADABS program specific to the CCD detector.27 The structure was solved by direct methods using SHELX-TL![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 28 and the hydrogen atoms were located using geometrical constraints. Refinement was performed by full-matrix least-squares analysis of SHELX-TL.
28 and the hydrogen atoms were located using geometrical constraints. Refinement was performed by full-matrix least-squares analysis of SHELX-TL.
| 6 | |
|---|---|
| Chemical formula | C12H8N3SF3O6 |
| Formula weight | 379.27 |
| Crystal system | monoclinic |
| Space group | P![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 21/c (no. 14) 21/c (no. 14) |
| Z | 4 |
| a/Å | 11.3081(6) |
| b/Å | 9.2398(5) |
| c/Å | 14.7668(8) |
| β/° | 111.5900(10) |
| V/Å3 | 1434.65(13) |
| T/K | 293(2) |
| Reflections collected | 10758 |
Independent reflections with I > 2σ(I![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) ) | 4261 |
| Rint | 0.0260 |
| μ/mm−1 | 0.302 |
| R1(Fo), wR2(Fo) | 0.052, 0.1717 |
| Bond | d/Å | Bond | d/Å |
|---|---|---|---|
| C(4)–C(5) | 1.511(3) | C(4)–C(9) | 1.501(3) |
| C(7)–C(10) | 1.561(3) | C(13)–C(14) | 1.494(3) |
| C(11)–C(12) | 1.308(4) | C(6)–C(7) | 1.529(3) |
| C(4)–N(2) | 1.508(2) | C(5)–C(6) | 1.327(3) |
| C(6)–S | 1.749(2) | N(4)–O(4b) | 1.181(3) |
| Bond | θ/° | Bond | θ/° |
|---|---|---|---|
| C(9)–C(4)–C(5) | 106.9(2) | C(11)–C(12)–C(13) | 112.9(2) |
| C(4)–C(5)–C(6) | 113.5(2) | C(10)–C(11)–C(12) | 104.0(2) |
| C(5)–C(6)–C(7) | 117.0(2) | C(7)–C(10)–C(11) | 110.5(2) |
| C(6)–C(7)–C(8) | 103.4(2) | C(7)–C(8)–C(9) | 116.2(2) |
CCDC reference number 188/199.
References
- (a) F. Terrier , in Nucleophilic Aromatic Displacement; The Influence of the Nitro Group, Organic Nitro Chem. Ser., ed. H. Feuer, VCH, New York, 1991, chapters 1 and 2; Search PubMed; (b) E. Buncel, J. M. Dust and F. Terrier, Chem. Rev., 1995, 95, 2261 CrossRef CAS; (c) F. Terrier, Chem. Rev., 1982, 82, 77 CrossRef CAS.
- (a) F. Terrier, F. Millot and W. P. Norris, J. Am. Chem. Soc., 1976, 98, 5883 CrossRef CAS; (b) F. Terrier, M. J. Pouet, E. Kizilian, J. C. Hallé, F. Outurquin and C. Paulmier, J. Org. Chem., 1993, 58, 4696 CrossRef CAS; (c) F. Terrier, E. Kizilian, J. C. Hallé and E. Buncel, J. Am. Chem. Soc., 1992, 114, 1740 CrossRef CAS and references therein; (d) (a) M. J. Strauss, R. A. Renfrow and E. Buncel, J. Am. Chem. Soc., 1983, 105, 2473 CrossRef CAS; (b) E. Buncel, R. A. Renfrow and M. J. Strauss, J. Org. Chem., 1987, 52, 488 CrossRef CAS; (c) E. Buncel, R. A. Manderville and J. M. Dust, J. Chem. Soc., Perkin Trans. 2, 1997, 1019 RSCand references therein.; (e) (a) R. J. Spear, W. P. Norris and R. W. Read, Tetrahedron Lett., 1983, 24, 1555 CrossRef CAS; (b) W. P. Norris, R. J. Spear and R. W. Read, Aust. J. Chem., 1983, 36, 297 CAS; (f) (a) J. Kind and H. J. Niclas, Synth. Commun., 1993, 23, 1569 CrossRef CAS; (b) H. J. Niclas and B. Göhrmann, Synth. Commun., 1989, 19, 2141 CAS; (c) H. J. Niclas, B. Göhrmann and E. Gründemann, Synth. Commun., 1989, 19, 2789 CrossRef CAS; (g) (a) P. B. Ghosh, B. Ternai and M. W. Whitehouse, Med. Res. Rev., 1981, 1, 159 CAS; (b) P. B. Ghosh and M. W. Whitehouse, J. Med. Chem., 1968, 11, 305 CrossRef CAS; (c) P. B. Ghosh and M. W. Whitehouse, J. Med. Chem., 1969, 12, 505 CrossRef CAS; (d) P. B. Ghosh and B. J. Everitt, J. Med. Chem., 1974, 17, 203 CrossRef CAS; (h) M. I. Evgen’yev, S. Y. Garmonov, I. I. Evgen’yeva and L. S. Gazizullina, J. Anal. Chem., 1998, 53, 571 Search PubMed and references therein.; (i) C. K. Lowe-Ma, R. A. Nissan and W. S. Wilson, J. Org. Chem., 1990, 55, 3755 CrossRef CAS; (j) (a) E. Kizilian, F. Terrier, A. P. Chatrousse, K. Gzouli and J. C. Hallé, J. Chem. Soc., Perkin Trans. 2, 1997, 2667 RSC; (b) F. Terrier, M. J. Pouet, J. C. Hallé, E. Kizilian and E. Buncel, J. Phys. Org. Chem., 1998, 11, 707 CrossRef CAS; (c) F. Terrier, M. J. Pouet, K. Gzouli, J. C. Hallé, F. Outurquin and C. Paulmier, Can. J. Chem., 1998, 76, 937 CrossRef CAS; (k) (a) J. H. Atherton, M. R. Crampton, G. L. Duffield and J. A. Stevens, J. Chem. Soc., Perkin Trans. 2, 1995, 443 RSC; (b) M. R. Crampton and L. C. Rabbit, J. Chem. Soc., Perkin Trans. 2, 1999, 1669 RSC; (l) G. Kresze and H. Bathelt, Tetrahedron, 1973, 29, 1043 CrossRef CAS; (m) (a) J. C. Hallé, D. Vichard, M. J. Pouet and F. Terrier, J. Org. Chem., 1997, 62, 7178 CrossRef CAS; (b) D. Vichard, J. C. Hallé, B. Huguet, M. J. Pouet, D. Riou and F. Terrier, Chem. Commun., 1998, 791 RSC; (c) P. Sepulcri, R. Goumont, J. C. Hallé, D. Riou and F. Terrier, J. Org. Chem., 1999, 64, 0 CrossRef CAS; (n) S. Pugnaud, D. Masure, J. C. Hallé and P. Chaquin, J. Org. Chem., 1997, 62, 8687 CrossRef CAS; (o) S. Weinreb and D. B. Boger , in Hetero Diels–Alder Methodology in Organic Synthesis, Academic Press, Orlando, 1987. Search PubMed; (p) (a) S. E. Denmark, B. S. Kesler and Y. C. Moon, J. Org. Chem., 1992, 57, 4912 CrossRef CAS; (b) S. E. Denmark and A. Thorarensen, Chem. Rev., 1996, 96, 137 CrossRef CASand references therein; (c) S. E. Denmark and A. Thorarensen, J. Am. Chem. Soc., 1997, 119, 127; (d) S. E. Denmark, R. A. Hurd and H. J. Sacha, J. Org. Chem., 1997, 62, 1668 CrossRef CAS; (e) S. E. Denmark and L. R. Marcin, J. Org. Chem., 1997, 62, 1675 CrossRef CAS; (q) W. A. Sheppard, J. Am. Chem. Soc., 1963, 85, 1314 CrossRef CAS; (r) F. G. Bordwell, N. R. Vanier, W. S. Matthews, J. B. Hendrickson and P. L. Skipper, J. Am. Chem. Soc., 1975, 97, 7160 CrossRef CAS; (s) F. Terrier, F. Millot and J. Morel, J. Org. Chem., 1976, 41, 3892 CrossRef CAS; (t) (a) L. M. Yagupol’skii, A. Ya. Ll’chenko and N. V. Kondratenko, Russ. Chem. Rev., 1974, 43, 32 Search PubMed; (b) J. M. Carpentier, F. Terrier, R. Schaal, N. V. Ignatev, V. N. Boiko and L. M. Yagupol’skii, Bull. Soc. Chim. Fr., 1985, 150 Search PubMed; (u) F. Terrier, E. Kizilian, R. Goumont, N. Faucher and C. Wakselman, J. Am. Chem. Soc., 1998, 120, 9496 CrossRef CAS; (v) G. Raabe, H. J. Gais and J. Fleischhauer, J. Am. Chem. Soc., 1996, 118, 4622 CrossRef CAS; (w) B. E. Smart , in Organofluorine Chemistry: Principles and Commercial Applications, ed. R. E. Banks, B. E. Smart and J. C. Tatlow, Plenum Press, New York, 1994, 81. Search PubMed; (x) (a) A. Ejchart, Org. Magn. Reson., 1977, 10, 263 Search PubMed; (b) F. Terrier, R. Goumont, M. J. Pouet and J. C. Hallé, J. Chem. Soc., Perkin Trans. 2, 1995, 1629 RSC; (y) P. Sepulcri , Ph.D. Thesis, University of Versailles, March 1999. Search PubMed; (z) L. M. Yagupol’skii, I. V. Gogoman, G. M. Shchupak and V. N. Boiko, Zh. Org. Khim., 1986, 22, 743 Search PubMed; (a a) B. Ghosh, B. Ternai and M. W. Whitehouse, J. Med. Chem., 1972, 15, 255 CrossRef; (a b) G. Sheldrick , SADABS program, Siemens Area Detector Absorption Corrections, Search PubMedunpublished..
- SHELXTL: Structure Analysis Program 5.04; Siemens Industrial Automation, Inc., Madison, WI, 1995. Search PubMed.
Footnote |
| † IUPAC name for benzofuroxan is benzofurazan N-oxide. |
| This journal is © The Royal Society of Chemistry 2000 |