A simple synthetic method for chiral 1,2-epoxides and the total synthesis of a chiral pheromone epoxide
Zhi-Bo
Zhang
,
Zhi-Min
Wang
*,
Yu-Xiu
Wang
,
Huan-Quan
Liu
,
Gui-Xin
Lei
and
Min
Shi
*
Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Lu, Shanghai, 200032, China
First published on 24th December 1999
Abstract
Chiral 1,2-epoxyalkan-3-ol tosyl esters 4a–h were successfully synthesized from alkynols or allyl chlorides in three steps using Sharpless AD reaction as a key step in good yields. The chiral insect pheromone epoxide (6Z,9S,10R)-9,10-epoxyhenicos-6-ene 9 was thus smoothly synthesized from the corresponding key intermediate 4g.
Introduction
Synthetic chemists always face a major challenge in the preparation of chiral compounds with high optical purity. The need for pure enantiomers is particularly apparent in the field of insect pheromone chemistry, since insect chemoreception can be highly stereoselective.1–3 Optically active epoxides are an important class of natural products encountered as sex attractants of Lepidopteran pests,![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 4 and self-defensive substances against rice blast disease.5 The optically active 1,2-epoxyalkan-3-ols are key intermediates in the synthesis of those insect pheromones because they can be easily converted to the corresponding optically active 2,3-epoxyalkan-1-ols through the Payne rearrangement
4 and self-defensive substances against rice blast disease.5 The optically active 1,2-epoxyalkan-3-ols are key intermediates in the synthesis of those insect pheromones because they can be easily converted to the corresponding optically active 2,3-epoxyalkan-1-ols through the Payne rearrangement![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 6 or to optically active internal epoxides via an alkylative rearrangement of the corresponding toluene-p-sulfonate (tosyl) esters.7 In order to obtain the chiral epoxides in those synthetic approaches, until now the mostly used key reaction has been the Sharpless AE reaction on the Z-allylic alcohols.7–9 Herein we report two further synthetic methods for the chiral 1,2-epoxyalkan-3-ol tosyl esters 4a–h using Sharpless AD
6 or to optically active internal epoxides via an alkylative rearrangement of the corresponding toluene-p-sulfonate (tosyl) esters.7 In order to obtain the chiral epoxides in those synthetic approaches, until now the mostly used key reaction has been the Sharpless AE reaction on the Z-allylic alcohols.7–9 Herein we report two further synthetic methods for the chiral 1,2-epoxyalkan-3-ol tosyl esters 4a–h using Sharpless AD![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 10 as the key reaction, and the total synthesis of the insect sex pheromone (6Z,9S,10R)-9,10-epoxyhenicos-6-ene 9 with full experimental details.11
10 as the key reaction, and the total synthesis of the insect sex pheromone (6Z,9S,10R)-9,10-epoxyhenicos-6-ene 9 with full experimental details.11
Results and discussion
Sharpless AD reaction on the starting materials 2 possessing different, long alkyl chains, prepared by reduction of the corresponding alkynols 1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 12 using LiAlH4 in THF, installed the two stereogenic centers, with 95–97% enantiomeric excess (ee).13 The resulting triols 3 were subsequently treated with NaH and Tos-Im
12 using LiAlH4 in THF, installed the two stereogenic centers, with 95–97% enantiomeric excess (ee).13 The resulting triols 3 were subsequently treated with NaH and Tos-Im![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 14 in THF to produce the key intermediates 4 in good yield (Scheme 1). To the best of our knowledge, this new synthetic approach is the shortest and the most efficient among those reported in previous literature.7–9 Thus 1,2-epoxy-3-tosyl esters (4a–h) with different alkyl chains were obtained as colorless solids or oils. Only one diastereoisomer was detected during this reaction. Their total yields in three steps, specific optical rotations and mps are summarized in Table 1. The chemical yields of 4a–h slightly increased with an increase in the alkyl chain length. The specific optical rotation of the key intermediate 4g was very close to that reported in the literature {lit.,8 [α]20D +8.3 (c 1, CHCl3)}.
14 in THF to produce the key intermediates 4 in good yield (Scheme 1). To the best of our knowledge, this new synthetic approach is the shortest and the most efficient among those reported in previous literature.7–9 Thus 1,2-epoxy-3-tosyl esters (4a–h) with different alkyl chains were obtained as colorless solids or oils. Only one diastereoisomer was detected during this reaction. Their total yields in three steps, specific optical rotations and mps are summarized in Table 1. The chemical yields of 4a–h slightly increased with an increase in the alkyl chain length. The specific optical rotation of the key intermediate 4g was very close to that reported in the literature {lit.,8 [α]20D +8.3 (c 1, CHCl3)}.
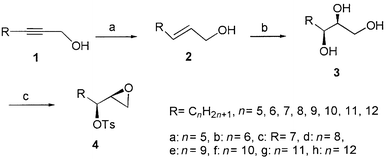 | ||
| Scheme 1 Reagents, conditions and yields: (a) LiAlH4, THF, reflux; 83–94%; (b) K3Fe(CN)6, K2CO3, NaHCO3, MeSO2NH2, (DHQ)2PHAL, K2OsO2(OH)4, tBuOH–H2O (1∶1), 0 °C; 68–89%; (c) NaH, Tos-Im, THF; 53–66%. | ||
In the meantime, another alternative synthetic procedure, which is very similar to that mentioned above, also can be utilized to the preparation of 4a–h using the corresponding allyl chlorides 5 as starting materials (Scheme 2). The chemical yields and reaction conditions are shown in Scheme 2 and the obtained chiral diols 6a–h with 94–97% ee![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 13 were directly transferred to the next reaction without purification to give epoxy tosyl esters 4 (step b, c) which have similar specific optical rotations and the same spectral data as those obtained in Scheme 1. The two synthetic routes are very convenient and useful for the synthesis of 4, and this clearly suggests that Sharpless AD is a very powerful and useful synthetic method for the construction ion of the chiral center on many substrates. Indeed, the chiral epoxides 4a–h can obviously be obtained by kinetic resolution using Sharpless AE reaction (Scheme 3). However, the direct asymmetric synthesis using Sharpless AD reaction is much more effective because all the starting materials could be transferred to the desired chiral compounds.
13 were directly transferred to the next reaction without purification to give epoxy tosyl esters 4 (step b, c) which have similar specific optical rotations and the same spectral data as those obtained in Scheme 1. The two synthetic routes are very convenient and useful for the synthesis of 4, and this clearly suggests that Sharpless AD is a very powerful and useful synthetic method for the construction ion of the chiral center on many substrates. Indeed, the chiral epoxides 4a–h can obviously be obtained by kinetic resolution using Sharpless AE reaction (Scheme 3). However, the direct asymmetric synthesis using Sharpless AD reaction is much more effective because all the starting materials could be transferred to the desired chiral compounds.
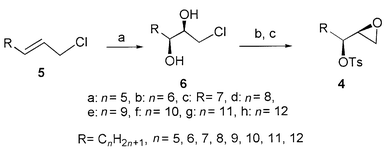 | ||
| Scheme 2 Reagents, conditions and yields: (a) K3Fe(CN)6, K2CO3, NaHCO3, MeSO2NH2, (DHQ)2PHAL, K2OsO2(OH)4, tBuOH–H2O (1∶1), 0 °C; 84–88%; (b) K2CO3, MeOH, rt; (c) NaH, Tos-Im, THF; 50–56% (two steps). | ||
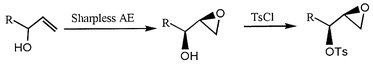 | ||
| Scheme 3 | ||
The synthesis of the sex pheromone of Phragmatobia fuliginosa is depicted in Scheme 4. The epoxide 4g was opened by 1-lithioheptyne in the presence of BF3·Et2O to afford compound 7. Treatment of 7 with K2CO3 in methanol gave another epoxide 8 in good yield. Catalytic hydrogenation of 8 over Lindlar catalyst easily gave the target compound (6Z,9S,10R)-9,10-epoxyhenicos-6-ene 9 in moderate yield. The specific optical rotation of our synthetic compound 9 is very close to those values reported in the literature {9: [α]20D +8.7 (c 0.97, CHCl3); lit.,15 [α]20D +9.4 (c 0.55, CHCl3)}. Its 1H NMR spectral data are completely consistent with those reported in the literature.15,16
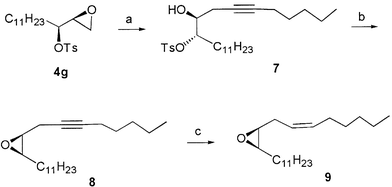 | ||
| Scheme 4 Reagents, conditions and yield: (a) Hept-1-yne, n-BuLi, BF3·OEt2, THF, −78 °C; 70%; (b) K2CO3, CH3OH, rt, 60%; (c) Pd–CaCO3, H2; 80%. | ||
In conclusion, we have developed two efficient and convenient procedures for the stereocontrolled synthesis of the chiral 1,2-epoxy-3-tosyl esters 4a–h from which the important chiral pheromone epoxide 9 has been successfully synthesized. This new synthetic approach using Sharpless AD reaction will certainly open a new and effective synthetic route to the preparation of those highly stereoselective chemoreception insect pheromones. In order to disclose the relationship between structure and biological activity, syntheses of their pheromone analogs are in progress.
Experimental
Mps were obtained with a Yanagimoto micro-melting-point apparatus and are uncorrected. Optical rotations were determined for solutions in CHCl3 or MeOH at 20 °C by using a Perkin-Elmer-241 MC digital polarimeter; [α]D-values are given in units of 10−1 deg cm2 g−1. 1H NMR spectra were determined for solutions in CDCl3 with tetramethylsilane (TMS) as internal standard on a Bruker AMX-300 spectrometer; J-values are in Hz. IR spectra were determined by a Perkin-Elmer 983 spectrometer. Mass spectra were recorded with an HP-5989 instrument. High-resolution mass spectra were recorded on a Finnigan MA+ instrument. All solid compounds reported in this paper gave satisfactory CHN microanalyses with an Italian Carlo-Erba 1106 analyzer. Petroleum spirit refers to the fraction with distillation range 60–70 °C. Hydroquinine phthalazine-1,4-diyl diether (DHQ)2PHAL was purchased from Aldrich.Typical reaction procedure for the preparation of E-allylic alcohols
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-Tetradec-2-en-1-ol 2g..
To a stirred solution of tetradec-2-yn-1-ol 1g (7.56 g, 36 mmol) in dry THF (100 ml) was added LiAlH4 (3.14 g, 82.6 mmol) slowly at 0 °C under nitrogen atmosphere. After stirring for 20 min, the mixture was heated under reflux for 6 h. The reaction was quenched by adding water at 0 °C. The mixture was filtered, and extracted with diethyl ether. The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by distillation under reduced pressure to afford 2g as a colorless liquid (7.47 g, 98%); bp 104 °C/1 mmHg; IR (neat) ν 3324, 2920, 1597, 1466 cm−1; 1H NMR (CDCl3) δ 0.89 (3H, t, J 6.6, CH3), 1.20–1.40 (18H, m, CH2), 1.95–2.10 (2H, m, CH2), 2.20–2.30 (1H, br s, OH), 4.1 (2H, d, J 5.0, CH2), 5.46–5.70 (2H, m, CH
)-Tetradec-2-en-1-ol 2g..
To a stirred solution of tetradec-2-yn-1-ol 1g (7.56 g, 36 mmol) in dry THF (100 ml) was added LiAlH4 (3.14 g, 82.6 mmol) slowly at 0 °C under nitrogen atmosphere. After stirring for 20 min, the mixture was heated under reflux for 6 h. The reaction was quenched by adding water at 0 °C. The mixture was filtered, and extracted with diethyl ether. The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by distillation under reduced pressure to afford 2g as a colorless liquid (7.47 g, 98%); bp 104 °C/1 mmHg; IR (neat) ν 3324, 2920, 1597, 1466 cm−1; 1H NMR (CDCl3) δ 0.89 (3H, t, J 6.6, CH3), 1.20–1.40 (18H, m, CH2), 1.95–2.10 (2H, m, CH2), 2.20–2.30 (1H, br s, OH), 4.1 (2H, d, J 5.0, CH2), 5.46–5.70 (2H, m, CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH); MS (EI) m/z 212 (M+) [Calc. for C14H28O (212.3715): C, 79.18; H, 13.29. Found: C, 79.12; H, 13.22%].
CH); MS (EI) m/z 212 (M+) [Calc. for C14H28O (212.3715): C, 79.18; H, 13.29. Found: C, 79.12; H, 13.22%].
Compounds 2a–f and 2h were prepared in the same manner to that described above.
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-Oct-2-en-1-ol 2a..
A colorless liquid (3.83 g, 83%); bp 88 °C/1 mmHg; IR (neat) ν 3324, 2923, 1669, 1466 cm−1; 1H NMR (CDCl3) δ 0.89 (3H, t, J 6.3, CH3), 1.20–1.40 (6H, m, CH2), 1.96–2.08 (2H, m, CH2), 1.80–1.90 (1H, br s, OH), 4.0 (2H, d, J 4.9, CH2), 5.43–5.60 (2H, m, CH
)-Oct-2-en-1-ol 2a..
A colorless liquid (3.83 g, 83%); bp 88 °C/1 mmHg; IR (neat) ν 3324, 2923, 1669, 1466 cm−1; 1H NMR (CDCl3) δ 0.89 (3H, t, J 6.3, CH3), 1.20–1.40 (6H, m, CH2), 1.96–2.08 (2H, m, CH2), 1.80–1.90 (1H, br s, OH), 4.0 (2H, d, J 4.9, CH2), 5.43–5.60 (2H, m, CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH); MS (EI) m/z 128 (M+) [Calc. for C8H16O (128.2120): C, 74.94; H, 12.58. Found: C, 74.86; H, 12.60%].
CH); MS (EI) m/z 128 (M+) [Calc. for C8H16O (128.2120): C, 74.94; H, 12.58. Found: C, 74.86; H, 12.60%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-Non-2-en-1-ol 2b..
A colorless liquid (4.78, g 93%); bp 90 °C/1 mmHg; IR (neat) ν 3323, 2924, 1704, 1468 cm−1; 1H NMR (CDCl3) δ 0.89 (3H, t, J 6.5, CH3), 1.20–1.42 (8H, m, CH2), 1.98–2.10 (2H, m, CH2), 2.60–2.70 (1H, br s, OH), 4.08 (2H, d, J 4.9, CH2), 5.40–5.60 (2H, m, CH
)-Non-2-en-1-ol 2b..
A colorless liquid (4.78, g 93%); bp 90 °C/1 mmHg; IR (neat) ν 3323, 2924, 1704, 1468 cm−1; 1H NMR (CDCl3) δ 0.89 (3H, t, J 6.5, CH3), 1.20–1.42 (8H, m, CH2), 1.98–2.10 (2H, m, CH2), 2.60–2.70 (1H, br s, OH), 4.08 (2H, d, J 4.9, CH2), 5.40–5.60 (2H, m, CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH); MS (EI) m/z 142 (M+) [Calc. for C9H18O (142.2386): C, 76.00; H, 12.76. Found: C, 75.87; H, 12.92%].
CH); MS (EI) m/z 142 (M+) [Calc. for C9H18O (142.2386): C, 76.00; H, 12.76. Found: C, 75.87; H, 12.92%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-Dec-2-en-1-ol 2c..
A colorless liquid (5.28 g, 94%); bp 94 °C/1 mmHg; IR (neat) ν 3326, 2925, 1667, 1463 cm−1; 1H NMR (CDCl3) δ 0.85 (3H, t, J 6.7, CH3), 1.20–1.48 (10H, m, CH2), 1.95–2.05 (2H, m, CH2), 2.40–2.80 (1H, br s, OH), 4.05 (2H, d, J 4.1, CH2), 5.45–5.70 (2H, m, CH
)-Dec-2-en-1-ol 2c..
A colorless liquid (5.28 g, 94%); bp 94 °C/1 mmHg; IR (neat) ν 3326, 2925, 1667, 1463 cm−1; 1H NMR (CDCl3) δ 0.85 (3H, t, J 6.7, CH3), 1.20–1.48 (10H, m, CH2), 1.95–2.05 (2H, m, CH2), 2.40–2.80 (1H, br s, OH), 4.05 (2H, d, J 4.1, CH2), 5.45–5.70 (2H, m, CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH); MS (EI) m/z 156 (M+) [Calc. for C10H20O (156.2652): C, 76.86; H, 12.90. Found: C, 76.90; H, 12.70%].
CH); MS (EI) m/z 156 (M+) [Calc. for C10H20O (156.2652): C, 76.86; H, 12.90. Found: C, 76.90; H, 12.70%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-Undec-2-en-1-ol 2d..
A colorless liquid (5.93 g, 92%); bp 98 °C/1 mmHg; IR (neat) ν 3330, 2924, 2853, 1669, 1462 cm−1; 1H NMR (CDCl3) δ 0.85 (3H, t, J 6.1, CH3), 1.20–1.40 (12H, m, CH2), 1.95–2.05 (2H, m, CH2), 2.40–2.80 (1H, br s, OH), 4.0 (2H, d, J 4.8, CH2), 5.45–5.70 (2H, m, CH
)-Undec-2-en-1-ol 2d..
A colorless liquid (5.93 g, 92%); bp 98 °C/1 mmHg; IR (neat) ν 3330, 2924, 2853, 1669, 1462 cm−1; 1H NMR (CDCl3) δ 0.85 (3H, t, J 6.1, CH3), 1.20–1.40 (12H, m, CH2), 1.95–2.05 (2H, m, CH2), 2.40–2.80 (1H, br s, OH), 4.0 (2H, d, J 4.8, CH2), 5.45–5.70 (2H, m, CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH); MS (EI) m/z 170 (M+) [Calc. for C11H22O (170.2918): C, 77.58; H, 13.02. Found: C, 77.54; H, 13.06%].
CH); MS (EI) m/z 170 (M+) [Calc. for C11H22O (170.2918): C, 77.58; H, 13.02. Found: C, 77.54; H, 13.06%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-Dodec-2-en-1-ol 2e..
A colorless liquid (6.16 g, 93%); bp 100 °C/1 mmHg; IR (neat) ν 3326, 2922, 2852, 1597, 1466 cm−1; 1H NMR (CDCl3) δ 0.85 (3H, t, J 6.7, CH3), 1.20–1.48 (14H, m, CH2), 1.60–1.80 (1H, br s, OH), 1.98–2.10 (2H, m, CH2), 4.05 (2H, d, J 5.05, CH2), 5.45–5.70 (2H, m, CH
)-Dodec-2-en-1-ol 2e..
A colorless liquid (6.16 g, 93%); bp 100 °C/1 mmHg; IR (neat) ν 3326, 2922, 2852, 1597, 1466 cm−1; 1H NMR (CDCl3) δ 0.85 (3H, t, J 6.7, CH3), 1.20–1.48 (14H, m, CH2), 1.60–1.80 (1H, br s, OH), 1.98–2.10 (2H, m, CH2), 4.05 (2H, d, J 5.05, CH2), 5.45–5.70 (2H, m, CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH); MS (EI) m/z 184 (M+) [Calc. for C12H24O (184.3184): C, 78.20; H, 13.12. Found: C, 78.12; H, 13.17%].
CH); MS (EI) m/z 184 (M+) [Calc. for C12H24O (184.3184): C, 78.20; H, 13.12. Found: C, 78.12; H, 13.17%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-Tridec-2-en-1-ol 2f..
A colorless liquid (6.42 g, 90%); bp 104 °C/1 mmHg; IR (neat) ν 3328, 2924, 2854, 1597, 1466 cm−1; 1H NMR (CDCl3) δ 0.88 (3H, t, J 6.6, CH3), 1.20–1.40 (16H, m, CH2), 2.05–2.12 (2H, m, CH2), 2.40–2.80 (1H, br s, OH), 4.10 (2H, d, J 5.0, CH2), 5.45–5.70 (2H, m, CH
)-Tridec-2-en-1-ol 2f..
A colorless liquid (6.42 g, 90%); bp 104 °C/1 mmHg; IR (neat) ν 3328, 2924, 2854, 1597, 1466 cm−1; 1H NMR (CDCl3) δ 0.88 (3H, t, J 6.6, CH3), 1.20–1.40 (16H, m, CH2), 2.05–2.12 (2H, m, CH2), 2.40–2.80 (1H, br s, OH), 4.10 (2H, d, J 5.0, CH2), 5.45–5.70 (2H, m, CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH); MS (EI) m/z 198 (M+) [Calc. for C13H26O (198.3449): C, 78.72; H, 13.21. Found: C, 78.54; H, 13.17%].
CH); MS (EI) m/z 198 (M+) [Calc. for C13H26O (198.3449): C, 78.72; H, 13.21. Found: C, 78.54; H, 13.17%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-Pentadec-2-en-1-ol 2h..
A colorless liquid (7.32 g, 90%); bp 140 °C/1 mmHg; IR (neat) ν 3326, 2926, 2855, 1597, 1464 cm−1; 1H NMR (CDCl3) δ 0.85 (3H, t, J 6.6, CH3), 1.20–1.40 (20H, m, CH2), 1.60–1.70 (1H, br s, OH), 2.05–2.12 (2H, m, CH2), 4.10 (2H, d, J 5.0, CH2), 5.45-5.70 (2H, m, CH
)-Pentadec-2-en-1-ol 2h..
A colorless liquid (7.32 g, 90%); bp 140 °C/1 mmHg; IR (neat) ν 3326, 2926, 2855, 1597, 1464 cm−1; 1H NMR (CDCl3) δ 0.85 (3H, t, J 6.6, CH3), 1.20–1.40 (20H, m, CH2), 1.60–1.70 (1H, br s, OH), 2.05–2.12 (2H, m, CH2), 4.10 (2H, d, J 5.0, CH2), 5.45-5.70 (2H, m, CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH); MS (EI) m/z 226 (M+) [Calc. for C15H30O (226.3981): C, 79.58; H, 13.36. Found: C, 79.50; H, 13.47%].
CH); MS (EI) m/z 226 (M+) [Calc. for C15H30O (226.3981): C, 79.58; H, 13.36. Found: C, 79.50; H, 13.47%].
Typical reaction procedure for the preparation of triols 3
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-Tetradecane-1,2,3-triol 3g..
To a stirred mixture of K2CO3 (0.83 mg, 6 mmol), K3Fe(CN)6 (2 g, 6 mmol), NaHCO3 (0.51 g, 6 mmol) and CH3SO2NH2 (0.2 mg, 2 mmol) in a mixture of 10 ml water and 10 ml t-BuOH were added (DHQ)2PHAL (16 mg, 0.02 mmol) and K2OSO2(OH)2 (7.5 mg, 0.02 mmol) and the reaction mixture was cooled to 0 °C. Compound 2g (424 mg, 2 mmol) was added into the reaction mixture, which was stirred for 6 h at 0 °C. The reaction was quenched by adding 3 g of anhydrous Na2SO3 at room temperature and the mixture was stirred for 30 min. After filtration the mixture was extracted with EtOAc several times, the combined extracts were washed successively with 5% HCl and brine, and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and the residue was purified by silica gel column chromatograph (eluent: petroleum spirit–EtOAc 1∶10) to obtain 3g as a white solid (443 mg, 90%); mp 82–83 °C; [α]D
−6.8 (c 1.0, CH3OH); IR (KBr) ν 3330, 2928, 1230, 563 cm−1; 1H NMR (CDCl3) δ 0.9 (3H, t, J 7.0, CH3), 1.15–1.40 (18H, m, CH2), 1.50–1.60 (2H, m, CH2), 2.12–2.30 (2H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.57 (1H, m), 3.60–3.90 (3H, m); MS (EI) m/z 247 (MH+), 229 (M − H2O) [Calc. for C14H30O3 (246.3862): C, 68.25; H, 12.27. Found: C, 68.20; H, 12.13%].
)-Tetradecane-1,2,3-triol 3g..
To a stirred mixture of K2CO3 (0.83 mg, 6 mmol), K3Fe(CN)6 (2 g, 6 mmol), NaHCO3 (0.51 g, 6 mmol) and CH3SO2NH2 (0.2 mg, 2 mmol) in a mixture of 10 ml water and 10 ml t-BuOH were added (DHQ)2PHAL (16 mg, 0.02 mmol) and K2OSO2(OH)2 (7.5 mg, 0.02 mmol) and the reaction mixture was cooled to 0 °C. Compound 2g (424 mg, 2 mmol) was added into the reaction mixture, which was stirred for 6 h at 0 °C. The reaction was quenched by adding 3 g of anhydrous Na2SO3 at room temperature and the mixture was stirred for 30 min. After filtration the mixture was extracted with EtOAc several times, the combined extracts were washed successively with 5% HCl and brine, and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and the residue was purified by silica gel column chromatograph (eluent: petroleum spirit–EtOAc 1∶10) to obtain 3g as a white solid (443 mg, 90%); mp 82–83 °C; [α]D
−6.8 (c 1.0, CH3OH); IR (KBr) ν 3330, 2928, 1230, 563 cm−1; 1H NMR (CDCl3) δ 0.9 (3H, t, J 7.0, CH3), 1.15–1.40 (18H, m, CH2), 1.50–1.60 (2H, m, CH2), 2.12–2.30 (2H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.57 (1H, m), 3.60–3.90 (3H, m); MS (EI) m/z 247 (MH+), 229 (M − H2O) [Calc. for C14H30O3 (246.3862): C, 68.25; H, 12.27. Found: C, 68.20; H, 12.13%].
Compounds 3a–f and 3h were prepared in the same manner to that described above.
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-Octane-1,2,3-triol 3a..
A white solid (220 mg, 68%); mp 62–64 °C; [α]D
−5.8 (c 1.0, CH3OH); IR (KBr) ν 3324, 2928, 1145, 546 cm−1; 1H NMR (CDCl3) δ 0.9 (3H, t, J 7.0, CH3),1.15–1.42 (6H, m, CH2), 1.50–1.60 (2H, m, CH2), 2.12–2.30 (2H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.60 (1H, m), 3.60–3.90 (3H, m); MS (EI) m/z 162 (M+), 144 (M − H2O) [Calc. for C8H18O3 (162.2267): C, 59.23; H, 11.18. Found: C, 59.12; H, 11.02%].
)-Octane-1,2,3-triol 3a..
A white solid (220 mg, 68%); mp 62–64 °C; [α]D
−5.8 (c 1.0, CH3OH); IR (KBr) ν 3324, 2928, 1145, 546 cm−1; 1H NMR (CDCl3) δ 0.9 (3H, t, J 7.0, CH3),1.15–1.42 (6H, m, CH2), 1.50–1.60 (2H, m, CH2), 2.12–2.30 (2H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.60 (1H, m), 3.60–3.90 (3H, m); MS (EI) m/z 162 (M+), 144 (M − H2O) [Calc. for C8H18O3 (162.2267): C, 59.23; H, 11.18. Found: C, 59.12; H, 11.02%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-Nonane-1,2,3-triol 3b..
A white solid (264 mg, 75%); mp 73–75 °C; [α]D
−6.0 (c 1.0, CH3OH); IR (KBr) ν 3335, 2928, 1230, 563 cm−1; 1H NMR (CDCl3) δ 0.88 (3H, t, J 7.0, CH3), 1.14–1.42 (8H, m, CH2), 1.47–1.58 (2H, m, CH2), 2.12–2.30 (2H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.45–3.58 (1H, m), 3.60–3.90 (3H, m); MS (EI) m/z 177 (MH+), 159 (MH − H2O) [Calc. for C9H20O3 (176.2533): C, 61.33; H, 11.44. Found: C, 61.52; H, 11.26%].
)-Nonane-1,2,3-triol 3b..
A white solid (264 mg, 75%); mp 73–75 °C; [α]D
−6.0 (c 1.0, CH3OH); IR (KBr) ν 3335, 2928, 1230, 563 cm−1; 1H NMR (CDCl3) δ 0.88 (3H, t, J 7.0, CH3), 1.14–1.42 (8H, m, CH2), 1.47–1.58 (2H, m, CH2), 2.12–2.30 (2H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.45–3.58 (1H, m), 3.60–3.90 (3H, m); MS (EI) m/z 177 (MH+), 159 (MH − H2O) [Calc. for C9H20O3 (176.2533): C, 61.33; H, 11.44. Found: C, 61.52; H, 11.26%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-Decane-1,2,3-triol 3c..
A white solid (297 mg, 78%); mp 76–77 °C; [α]D
−6.2 (c 1.0, CH3OH); IR (KBr) ν 3341, 2928, 1225, 569 cm−1; 1H NMR (CDCl3) δ 0.88 (3H, t, J 7.0, CH3), 1.14–1.42 (10H, m, CH2), 1.47–1.60 (2H, m, CH2), 2.12–2.30 (2H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.48–3.58 (1H, m), 3.60–3.90 (3H, m); MS (EI) m/z 191 (MH+), 173 (MH − H2O) [Calc. for C10H22O3 (190.2799): C, 63.12; H, 11.65. Found: C, 63.22; H, 11.67%].
)-Decane-1,2,3-triol 3c..
A white solid (297 mg, 78%); mp 76–77 °C; [α]D
−6.2 (c 1.0, CH3OH); IR (KBr) ν 3341, 2928, 1225, 569 cm−1; 1H NMR (CDCl3) δ 0.88 (3H, t, J 7.0, CH3), 1.14–1.42 (10H, m, CH2), 1.47–1.60 (2H, m, CH2), 2.12–2.30 (2H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.48–3.58 (1H, m), 3.60–3.90 (3H, m); MS (EI) m/z 191 (MH+), 173 (MH − H2O) [Calc. for C10H22O3 (190.2799): C, 63.12; H, 11.65. Found: C, 63.22; H, 11.67%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-Undecane-1,2,3-triol 3d..
A white solid (322 mg, 79%); mp 79–80 °C; [α]D
−6.8 (c 1.3, CH3OH); IR (KBr) ν 3330, 2928, 1250, 543 cm−1; 1H NMR (CDCl3) δ 0.90 (3H, t, J 7.0, CH3), 1.14–1.40 (12H, m, CH2), 1.50–1.56 (2H, m, CH2), 2.12–2.30 (2H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.58 (1H, m), 3.60–3.92 (3H, m); MS (EI) m/z 205 (MH+), 187 (MH − H2O) [Calc. for C11H24O3 (204.3065): C, 64.67; H, 11.84. Found: C, 64.52; H, 11.87%].
)-Undecane-1,2,3-triol 3d..
A white solid (322 mg, 79%); mp 79–80 °C; [α]D
−6.8 (c 1.3, CH3OH); IR (KBr) ν 3330, 2928, 1250, 543 cm−1; 1H NMR (CDCl3) δ 0.90 (3H, t, J 7.0, CH3), 1.14–1.40 (12H, m, CH2), 1.50–1.56 (2H, m, CH2), 2.12–2.30 (2H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.58 (1H, m), 3.60–3.92 (3H, m); MS (EI) m/z 205 (MH+), 187 (MH − H2O) [Calc. for C11H24O3 (204.3065): C, 64.67; H, 11.84. Found: C, 64.52; H, 11.87%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-Dodecane-1,2,3-triol 3e..
A white solid (349 mg, 84%); mp 79–80 °C; [α]D
−6.8 (c 1.0, CH3OH); IR (KBr) ν 3328, 2928, 1267, 547 cm−1; 1H NMR (CDCl3) δ 0.89 (3H, t, J 7.0, CH3), 1.14–1.42 (14H, m, CH2), 1.50–1.56 (2H, m, CH2), 2.12–2.30 (2H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.58 (1H, m), 3.60–3.92 (3H, m); MS (EI) m/z 219 (MH+), 201 (MH − H2O) [Calc. for C12H26O3 (218.3330): C, 66.01; H, 12.00. Found: C, 66.22; H, 12.11%].
)-Dodecane-1,2,3-triol 3e..
A white solid (349 mg, 84%); mp 79–80 °C; [α]D
−6.8 (c 1.0, CH3OH); IR (KBr) ν 3328, 2928, 1267, 547 cm−1; 1H NMR (CDCl3) δ 0.89 (3H, t, J 7.0, CH3), 1.14–1.42 (14H, m, CH2), 1.50–1.56 (2H, m, CH2), 2.12–2.30 (2H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.58 (1H, m), 3.60–3.92 (3H, m); MS (EI) m/z 219 (MH+), 201 (MH − H2O) [Calc. for C12H26O3 (218.3330): C, 66.01; H, 12.00. Found: C, 66.22; H, 12.11%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-Tridecane-1,2,3-triol 3f..
A white solid (408 mg, 88%); mp 80–81 °C; [α]D
−7.0 (c 1.3, CH3OH); IR (KBr) ν 3330, 2928, 1272, 551 cm−1; 1H NMR (CDCl3) δ 0.88 (3H, t, J 7.0, CH3), 1.14–1.40 (16H, m, CH2), 1.50–1.58 (2H, m, CH2), 2.12–2.30 (2H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.58 (1H, m), 3.62–3.90 (3H, m); MS (EI) m/z 233 (MH+), 215 (MH − H2O) [Calc. for C13H28O3 (232.3596): C, 67.20; H, 12.15. Found: C, 67.08; H, 12.27%].
)-Tridecane-1,2,3-triol 3f..
A white solid (408 mg, 88%); mp 80–81 °C; [α]D
−7.0 (c 1.3, CH3OH); IR (KBr) ν 3330, 2928, 1272, 551 cm−1; 1H NMR (CDCl3) δ 0.88 (3H, t, J 7.0, CH3), 1.14–1.40 (16H, m, CH2), 1.50–1.58 (2H, m, CH2), 2.12–2.30 (2H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.58 (1H, m), 3.62–3.90 (3H, m); MS (EI) m/z 233 (MH+), 215 (MH − H2O) [Calc. for C13H28O3 (232.3596): C, 67.20; H, 12.15. Found: C, 67.08; H, 12.27%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-Pentadecane-1,2,3-triol 3h..
A white solid (438 mg, 89%); mp 85–87 °C; [α]D
−7.0 (c 1.0, CH3OH); IR (KBr) ν 3329, 2927, 1230, 562 cm−1; 1H NMR (CDCl3) δ 0.92 (3H, t, J 7.0, CH3), 1.14–1.40 (20H, m, CH2), 1.52–1.62 (2H, m, CH2), 2.12–2.30 (2H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.57 (1H, m), 3.60–3.90 (3H, m); MS (EI) m/z 247 (MH+), 229 (MH − H2O) [Calc. for C15H32O3 (260.4128): C, 69.18; H, 12.39. Found: C, 69.22; H, 12.37%].
)-Pentadecane-1,2,3-triol 3h..
A white solid (438 mg, 89%); mp 85–87 °C; [α]D
−7.0 (c 1.0, CH3OH); IR (KBr) ν 3329, 2927, 1230, 562 cm−1; 1H NMR (CDCl3) δ 0.92 (3H, t, J 7.0, CH3), 1.14–1.40 (20H, m, CH2), 1.52–1.62 (2H, m, CH2), 2.12–2.30 (2H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.57 (1H, m), 3.60–3.90 (3H, m); MS (EI) m/z 247 (MH+), 229 (MH − H2O) [Calc. for C15H32O3 (260.4128): C, 69.18; H, 12.39. Found: C, 69.22; H, 12.37%].
Typical reaction procedure for the preparation of 1,2-chiral epoxides 4
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1,2-Epoxy-3-(tosyloxy)tetradecane 4g..
To a solution of 3g (246 mg, 1 mmol) in dry THF was added 95% NaH (72 mg, 3 mmol) and the mixture was stirred for 30 min at room temperature. Then Tos-Im was added into the reaction solution, which was further stirred for 6 h before being poured into ice–water and extracted with EtOAc. The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatograph (eluent: EtOAc–petroleum spirit 1∶6) to give compound 4g as a colorless solid (241 mg, 63%); mp 71–72 °C, [α]D +8.6 (c 1, CHCl3); IR (KBr) ν 3202, 1599, 1350 cm−1; 1H NMR (CDCl3) δ 0.90 (3H, t, J 6.5, CH3), 1.25–1.50 (18H, m, CH2), 1.65–1.85 (2H, m, CH2), 2.45 (3H, s, CH3), 2.60 (1H, dd, J 5.0, 2.5), 2.78 (1H, t, J 5.0), 3.05–3.12 (1H, m), 4.35 (1H, q, J 6.0, CH), 7.35 (2H, d, J 7.5, ArH), 7.8 (2H, d, J 7.5, ArH); MS (EI) m/z 383 (MH+), 227 (M+ − C11H23) [Calc. for C21H34O4S (382.5583): C, 65.93; H, 8.96. Found: C, 65.82; H, 8.97%].
)-1,2-Epoxy-3-(tosyloxy)tetradecane 4g..
To a solution of 3g (246 mg, 1 mmol) in dry THF was added 95% NaH (72 mg, 3 mmol) and the mixture was stirred for 30 min at room temperature. Then Tos-Im was added into the reaction solution, which was further stirred for 6 h before being poured into ice–water and extracted with EtOAc. The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatograph (eluent: EtOAc–petroleum spirit 1∶6) to give compound 4g as a colorless solid (241 mg, 63%); mp 71–72 °C, [α]D +8.6 (c 1, CHCl3); IR (KBr) ν 3202, 1599, 1350 cm−1; 1H NMR (CDCl3) δ 0.90 (3H, t, J 6.5, CH3), 1.25–1.50 (18H, m, CH2), 1.65–1.85 (2H, m, CH2), 2.45 (3H, s, CH3), 2.60 (1H, dd, J 5.0, 2.5), 2.78 (1H, t, J 5.0), 3.05–3.12 (1H, m), 4.35 (1H, q, J 6.0, CH), 7.35 (2H, d, J 7.5, ArH), 7.8 (2H, d, J 7.5, ArH); MS (EI) m/z 383 (MH+), 227 (M+ − C11H23) [Calc. for C21H34O4S (382.5583): C, 65.93; H, 8.96. Found: C, 65.82; H, 8.97%].
Compounds 4a–f and 4h were prepared in the same manner to that described above.
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1,2-Epoxy-3-(tosyloxy)octane 4a..
A colorless oil (158 mg, 53%); [α]D +8.5 (c 1.0, CHCl3); IR (KBr) ν 3200, 1597, 1350 cm−1; 1H NMR (CDCl3) δ0.92 (3H, t, J 6.5, CH3), 1.25–1.50 (6H, m, CH2), 1.65–1.85 (2H, m, CH2), 2.44 (3H, s, CH3), 2.65 (1H, dd, J 4.6, 2.5, CH), 2.75 (1H, t, J 4.5, CH), 3.0–3.10 (1H, m, CH), 4.35 (1H, q, J 6.0), 7.35 (2H, d, J 7.5, ArH), 7.85 (2H, d, J 7.5, ArH); MS (EI) m/z 299 (MH+) [Calc. for C15H22O4S (298.3988): C, 60.38; H, 7.43. Found: C, 60.52; H, 7.33%].
)-1,2-Epoxy-3-(tosyloxy)octane 4a..
A colorless oil (158 mg, 53%); [α]D +8.5 (c 1.0, CHCl3); IR (KBr) ν 3200, 1597, 1350 cm−1; 1H NMR (CDCl3) δ0.92 (3H, t, J 6.5, CH3), 1.25–1.50 (6H, m, CH2), 1.65–1.85 (2H, m, CH2), 2.44 (3H, s, CH3), 2.65 (1H, dd, J 4.6, 2.5, CH), 2.75 (1H, t, J 4.5, CH), 3.0–3.10 (1H, m, CH), 4.35 (1H, q, J 6.0), 7.35 (2H, d, J 7.5, ArH), 7.85 (2H, d, J 7.5, ArH); MS (EI) m/z 299 (MH+) [Calc. for C15H22O4S (298.3988): C, 60.38; H, 7.43. Found: C, 60.52; H, 7.33%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1,2-Epoxy-3-(tosyloxy)nonane 4b..
A colorless oil (172 mg, 55%); [α]D +8.2 (c 1.0, CHCl3); IR (KBr) ν 3200, 1597, 1350 cm−1; 1H NMR (CDCl3) δ0.92 (3H, t, J 6.5, CH3), 1.25–1.50 (8H, m, CH2), 1.65–1.85 (2H, m, CH2), 2.43 (3H, s, CH3), 2.65 (1H, dd, J 4.6, 2.5, CH), 2.75 (1H, t, J 4.5, CH), 3.0–3.10 (1H, m, CH), 4.35 (1H, q, J 6.0, 15.0, CH), 7.35 (2H, d, J 7.6, ArH), 7.85 (2H, d, J 7.5, ArH); MS (EI) m/z 313 (MH+) [Calc. for C16H24O4S (312.4254): C, 61.51; H, 7.74. Found: C, 61.46; H, 7.70%].
)-1,2-Epoxy-3-(tosyloxy)nonane 4b..
A colorless oil (172 mg, 55%); [α]D +8.2 (c 1.0, CHCl3); IR (KBr) ν 3200, 1597, 1350 cm−1; 1H NMR (CDCl3) δ0.92 (3H, t, J 6.5, CH3), 1.25–1.50 (8H, m, CH2), 1.65–1.85 (2H, m, CH2), 2.43 (3H, s, CH3), 2.65 (1H, dd, J 4.6, 2.5, CH), 2.75 (1H, t, J 4.5, CH), 3.0–3.10 (1H, m, CH), 4.35 (1H, q, J 6.0, 15.0, CH), 7.35 (2H, d, J 7.6, ArH), 7.85 (2H, d, J 7.5, ArH); MS (EI) m/z 313 (MH+) [Calc. for C16H24O4S (312.4254): C, 61.51; H, 7.74. Found: C, 61.46; H, 7.70%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1,2-Epoxy-3-(tosyloxy)decane 4c..
A white solid (186 mg, 57%); mp 54–55 °C; [α]D +8.5 (c 1.0, CHCl3); IR (KBr) ν 3205, 1597, 1350 cm−1; 1H NMR (CDCl3) δ 0.9 (3H, t, J 6.5, CH3), 1.25–1.50 (10H, m, CH2), 1.65–1.85 (2H, m, CH2), 2.45 (3H, s, CH3), 2.65 (1H, dd, J 4.6, 2.6, CH), 2.78 (1H, t, J 4.6, CH), 3.10 (1H, m, CH), 4.35 (1H, q, J 6.0, 15.0, CH), 7.35 (2H, d, J 7.5, ArH), 7.85 (2H, d, J 7.5, ArH); MS (EI) m/z 327 (MH+) [Calc. for C17H26O4S (326.4519): C, 62.55; H, 8.03. Found: C, 62.52 ; H, 8.17%].
)-1,2-Epoxy-3-(tosyloxy)decane 4c..
A white solid (186 mg, 57%); mp 54–55 °C; [α]D +8.5 (c 1.0, CHCl3); IR (KBr) ν 3205, 1597, 1350 cm−1; 1H NMR (CDCl3) δ 0.9 (3H, t, J 6.5, CH3), 1.25–1.50 (10H, m, CH2), 1.65–1.85 (2H, m, CH2), 2.45 (3H, s, CH3), 2.65 (1H, dd, J 4.6, 2.6, CH), 2.78 (1H, t, J 4.6, CH), 3.10 (1H, m, CH), 4.35 (1H, q, J 6.0, 15.0, CH), 7.35 (2H, d, J 7.5, ArH), 7.85 (2H, d, J 7.5, ArH); MS (EI) m/z 327 (MH+) [Calc. for C17H26O4S (326.4519): C, 62.55; H, 8.03. Found: C, 62.52 ; H, 8.17%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1,2-Epoxy-3-(tosyloxy)undecane 4d..
A white solid (204 mg, 60%); mp 57–58 °C; [α]D +8.0 (c 1.0, CHCl3); IR (KBr) ν 3205, 1597, 1350 cm−1; 1H NMR (CDCl3) δ 0.9 (3H, t, J 6.5, CH3), 1.25–1.50 (12H, m, CH2), 1.65–1.85 (2H, m, CH2), 2.45 (3H, s, CH3), 2.65 (1H, dd, J 4.6, 2.6, CH), 2.78 (1H, t, J 4.6, CH), 3.0–3.10 (1H, m, CH), 4.35 (1H, q, J 6.0, CH), 7.35 (2H, d, J 7.5, ArH), 7.85 (2H, d, J 7.5, ArH); MS (EI) m/z 341 (MH+) [Calc. for C18H28O4S (340.4785): C, 63.50; H, 8.29. Found: C, 63.72; H, 8.07%].
)-1,2-Epoxy-3-(tosyloxy)undecane 4d..
A white solid (204 mg, 60%); mp 57–58 °C; [α]D +8.0 (c 1.0, CHCl3); IR (KBr) ν 3205, 1597, 1350 cm−1; 1H NMR (CDCl3) δ 0.9 (3H, t, J 6.5, CH3), 1.25–1.50 (12H, m, CH2), 1.65–1.85 (2H, m, CH2), 2.45 (3H, s, CH3), 2.65 (1H, dd, J 4.6, 2.6, CH), 2.78 (1H, t, J 4.6, CH), 3.0–3.10 (1H, m, CH), 4.35 (1H, q, J 6.0, CH), 7.35 (2H, d, J 7.5, ArH), 7.85 (2H, d, J 7.5, ArH); MS (EI) m/z 341 (MH+) [Calc. for C18H28O4S (340.4785): C, 63.50; H, 8.29. Found: C, 63.72; H, 8.07%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1,2-Epoxy-3-(tosyloxy)dodecane 4e..
A white solid (205 mg, 58%); mp 59–60 °C; [α]D +8.7 (c 1.0, CHCl3); IR (KBr) ν 3205, 1597, 1350 cm−1; 1H NMR (CDCl3) δ0.9 (3H, t, J 6.5, CH3), 1.25–1.50 (14H, m, CH2), 1.70–1.90 (2H, m, CH2), 2.45 (3H, s, CH3), 2.65 (1H, dd, J 4.6, 2.5, CH), 2.75 (1H, t, J 4.5, CH), 3.0-3.10 (1H, m, CH), 4.35 (1H, q, J 6.0, CH), 7.35 (2H, d, J 7.5, ArH), 7.85 (2H, d, J 7.5, ArH); MS (EI) m/z 355 (MH+) [Calc. for C19H30O4S (354.5051): C, 64.37; H, 8.53. Found: C, 64.33; H, 8.67%].
)-1,2-Epoxy-3-(tosyloxy)dodecane 4e..
A white solid (205 mg, 58%); mp 59–60 °C; [α]D +8.7 (c 1.0, CHCl3); IR (KBr) ν 3205, 1597, 1350 cm−1; 1H NMR (CDCl3) δ0.9 (3H, t, J 6.5, CH3), 1.25–1.50 (14H, m, CH2), 1.70–1.90 (2H, m, CH2), 2.45 (3H, s, CH3), 2.65 (1H, dd, J 4.6, 2.5, CH), 2.75 (1H, t, J 4.5, CH), 3.0-3.10 (1H, m, CH), 4.35 (1H, q, J 6.0, CH), 7.35 (2H, d, J 7.5, ArH), 7.85 (2H, d, J 7.5, ArH); MS (EI) m/z 355 (MH+) [Calc. for C19H30O4S (354.5051): C, 64.37; H, 8.53. Found: C, 64.33; H, 8.67%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )–1,2-Epoxy-3-(tosyloxy)tridecane 4f..
A white solid (206 mg, 56%); mp 72–74 °C; [α]D +8.3 (c 1.0, CHCl3); IR (KBr) ν 3205, 1597, 1350 cm−1; 1H NMR (CDCl3) δ 0.9 (3H, t, J 6.5, CH3), 1.27–1.52 (16H, m, CH2), 1.70–1.90 (2H, m, CH2), 2.43 (3H, s, CH3), 2.65 (1H, dd, J 4.6, 2.5, CH), 2.75 (1H, t, J 4.5, CH), 3.0–3.10 (1H, m, CH), 4.35 (1H, q, J 6.0, CH), 7.35 (2H, d, J 7.5, ArH), 7.85 (2H, d, J 7.5, ArH); MS (EI) m/z 369 (MH+) [Calc. for C20H32O4S (368.5317): C, 65.18; H, 8.75. Found: C, 65.11; H, 8.91%].
)–1,2-Epoxy-3-(tosyloxy)tridecane 4f..
A white solid (206 mg, 56%); mp 72–74 °C; [α]D +8.3 (c 1.0, CHCl3); IR (KBr) ν 3205, 1597, 1350 cm−1; 1H NMR (CDCl3) δ 0.9 (3H, t, J 6.5, CH3), 1.27–1.52 (16H, m, CH2), 1.70–1.90 (2H, m, CH2), 2.43 (3H, s, CH3), 2.65 (1H, dd, J 4.6, 2.5, CH), 2.75 (1H, t, J 4.5, CH), 3.0–3.10 (1H, m, CH), 4.35 (1H, q, J 6.0, CH), 7.35 (2H, d, J 7.5, ArH), 7.85 (2H, d, J 7.5, ArH); MS (EI) m/z 369 (MH+) [Calc. for C20H32O4S (368.5317): C, 65.18; H, 8.75. Found: C, 65.11; H, 8.91%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1,2-Epoxy-3-(tosyloxy)pentadecane 4h..
A white solid (262 mg, 66%); mp 85–86 °C; [α]D +8.0 (c 1.0, CHCl3); IR (KBr) ν 3205, 1597, 1350 cm−1; 1H NMR (CDCl3) δ0.9 (3H, t, J 6.5, CH3), 1.25–1.50 (20H, m, CH2), 1.65–1.85 (2H, m, CH2), 2.43 (3H, s, CH3), 2.60 (1H, dd, J 5.0, 2.5, CH), 2.78 (1H, t, J 4.5, CH), 3.0–3.10 (1H, m, CH), 4.35 (1H, q, J 5.0, CH), 7.35 (2H, d, J 7.5, ArH), 7.85 (2H, d, J 7.5, ArH); MS (EI) m/z 397 (MH+) [Calc. for C22H36O4S (396.5848): C, 66.63; H, 9.15. Found: C, 66.78; H, 9.27%].
)-1,2-Epoxy-3-(tosyloxy)pentadecane 4h..
A white solid (262 mg, 66%); mp 85–86 °C; [α]D +8.0 (c 1.0, CHCl3); IR (KBr) ν 3205, 1597, 1350 cm−1; 1H NMR (CDCl3) δ0.9 (3H, t, J 6.5, CH3), 1.25–1.50 (20H, m, CH2), 1.65–1.85 (2H, m, CH2), 2.43 (3H, s, CH3), 2.60 (1H, dd, J 5.0, 2.5, CH), 2.78 (1H, t, J 4.5, CH), 3.0–3.10 (1H, m, CH), 4.35 (1H, q, J 5.0, CH), 7.35 (2H, d, J 7.5, ArH), 7.85 (2H, d, J 7.5, ArH); MS (EI) m/z 397 (MH+) [Calc. for C22H36O4S (396.5848): C, 66.63; H, 9.15. Found: C, 66.78; H, 9.27%].
The preparation of diols 6
The reaction procedure is the same as that described in the preparation of diol 3, but starting from the E-allyl chlorides 5.![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1-Chlorooctane-2,3-diol 6a..
A white solid (302 mg, 84%); mp 76–78 °C; [α]D
−9.5 (c 1.0, CH3OH); IR (KBr) ν 3324, 2928, 1145, 546 cm−1; 1H NMR (CDCl3) δ 0.9 (3H, t, J 7.0, CH3), 1.15–1.42 (6H, m, CH2), 1.50–1.60 (2H, m, CH2), 2.12–2.30 (1H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.48–3.60 (2H, m), 3.60–3.96 (2H, m); MS (EI) m/z 178, 180 (M+), 162 (M − H2O) [Calc. for C8H17ClO2 (180.6721): C, 53.18; H, 9.48. Found: C, 53.02; H, 9.32%].
)-1-Chlorooctane-2,3-diol 6a..
A white solid (302 mg, 84%); mp 76–78 °C; [α]D
−9.5 (c 1.0, CH3OH); IR (KBr) ν 3324, 2928, 1145, 546 cm−1; 1H NMR (CDCl3) δ 0.9 (3H, t, J 7.0, CH3), 1.15–1.42 (6H, m, CH2), 1.50–1.60 (2H, m, CH2), 2.12–2.30 (1H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.48–3.60 (2H, m), 3.60–3.96 (2H, m); MS (EI) m/z 178, 180 (M+), 162 (M − H2O) [Calc. for C8H17ClO2 (180.6721): C, 53.18; H, 9.48. Found: C, 53.02; H, 9.32%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1-Chlorononane-2,3-diol 6b..
A white solid (330 mg, 85%); mp 82–84 °C; [α]D
−9.0 (c 1.0, CH3OH); IR (KBr) ν 3335, 2928, 1230, 563 cm−1; 1H NMR (CDCl3) δ 0.88 (3H, t, J 7.0, CH3), 1.14–1.42 (8H, m, CH2), 1.47–1.58 (2H, m, CH2), 2.12–2.30 (1H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.45–3.60 (2H, m), 3.70–3.97 (2H, m); MS (EI) m/z 192, 194 (M+), 176 (M − H2O) [Calc. for C9H19ClO2 (194.6987): C, 55.52; H, 9.84. Found: C, 55.62; H, 9.98%].
)-1-Chlorononane-2,3-diol 6b..
A white solid (330 mg, 85%); mp 82–84 °C; [α]D
−9.0 (c 1.0, CH3OH); IR (KBr) ν 3335, 2928, 1230, 563 cm−1; 1H NMR (CDCl3) δ 0.88 (3H, t, J 7.0, CH3), 1.14–1.42 (8H, m, CH2), 1.47–1.58 (2H, m, CH2), 2.12–2.30 (1H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.45–3.60 (2H, m), 3.70–3.97 (2H, m); MS (EI) m/z 192, 194 (M+), 176 (M − H2O) [Calc. for C9H19ClO2 (194.6987): C, 55.52; H, 9.84. Found: C, 55.62; H, 9.98%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1-Chlorodecane-2,3-diol 6c..
A white solid (363 mg, 88%); mp 86–87 °C; [α]D
−9.6 (c 1.0, CH3OH); IR (KBr) ν 3341, 2928, 1225, 569 cm−1; 1H NMR (CDCl3) δ 0.88 (3H, t, J 7.0, CH3), 1.14–1.42 (10H, m, CH2), 1.47–1.60 (2H, m, CH2), 2.12–2.30 (1H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.48–3.58 (2H, m), 3.70–4.0 (2H, m); MS (EI) m/z 206, 208 (M+), 192 (M − H2O) [Calc. for C10H21ClO2 (208.7252): C, 57.54; H, 10.14. Found: C, 57.29; H, 10.27%].
)-1-Chlorodecane-2,3-diol 6c..
A white solid (363 mg, 88%); mp 86–87 °C; [α]D
−9.6 (c 1.0, CH3OH); IR (KBr) ν 3341, 2928, 1225, 569 cm−1; 1H NMR (CDCl3) δ 0.88 (3H, t, J 7.0, CH3), 1.14–1.42 (10H, m, CH2), 1.47–1.60 (2H, m, CH2), 2.12–2.30 (1H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.48–3.58 (2H, m), 3.70–4.0 (2H, m); MS (EI) m/z 206, 208 (M+), 192 (M − H2O) [Calc. for C10H21ClO2 (208.7252): C, 57.54; H, 10.14. Found: C, 57.29; H, 10.27%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1-Chloroundecane-2,3-diol 6d..
A white solid (382 mg, 86%); mp 87–89 °C; [α]D
−9.6 (c 1.3, CH3OH); IR (KBr) ν 3330, 2928,1250, 543 cm−1; 1H NMR (CDCl3) δ 0.90 (3H, t, J 7.0, CH3), 1.14–1.40 (12H, m, CH2), 1.50–1.56 (2H, m, CH2), 2.12–2.30 (1H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.47–3.58 (2H, m), 3.70–3.97 (2H, m); MS (EI) m/z 220, 222 (M+), 204 (M − H2O) [Calc. for C11H23ClO2 (222.7518): C, 59.31; H, 10.41. Found: C, 59.42; H, 10.67%].
)-1-Chloroundecane-2,3-diol 6d..
A white solid (382 mg, 86%); mp 87–89 °C; [α]D
−9.6 (c 1.3, CH3OH); IR (KBr) ν 3330, 2928,1250, 543 cm−1; 1H NMR (CDCl3) δ 0.90 (3H, t, J 7.0, CH3), 1.14–1.40 (12H, m, CH2), 1.50–1.56 (2H, m, CH2), 2.12–2.30 (1H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.47–3.58 (2H, m), 3.70–3.97 (2H, m); MS (EI) m/z 220, 222 (M+), 204 (M − H2O) [Calc. for C11H23ClO2 (222.7518): C, 59.31; H, 10.41. Found: C, 59.42; H, 10.67%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1-Chlorododecane-2,3-diol 6e..
A white solid (394 mg, 84%); mp 89–90 °C; [α]D
−9.8 (c 1.0, CH3OH); IR (KBr) ν 3328, 2928, 1267, 547 cm−1; 1H NMR (CDCl3) δ 0.89 (3H, t, J 7.0, CH3), 1.14–1.42 (14H, m, CH2), 1.50–1.56 (2H, m, CH2), 2.12–2.30 (1H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.62 (2H, m), 3.70–3.98 (2H, m); MS (EI) m/z 234, 236 (M+), 218 (M − H2O) [Calc. for C12H25ClO2 (236.7784): C, 60.87; H, 10.64. Found: C, 60.72; H, 10.61%].
)-1-Chlorododecane-2,3-diol 6e..
A white solid (394 mg, 84%); mp 89–90 °C; [α]D
−9.8 (c 1.0, CH3OH); IR (KBr) ν 3328, 2928, 1267, 547 cm−1; 1H NMR (CDCl3) δ 0.89 (3H, t, J 7.0, CH3), 1.14–1.42 (14H, m, CH2), 1.50–1.56 (2H, m, CH2), 2.12–2.30 (1H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.62 (2H, m), 3.70–3.98 (2H, m); MS (EI) m/z 234, 236 (M+), 218 (M − H2O) [Calc. for C12H25ClO2 (236.7784): C, 60.87; H, 10.64. Found: C, 60.72; H, 10.61%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1-Chlorotridecane-2,3-diol 6f..
A white solid (440 mg, 88%); mp 90–91 °C; [α]D
−9.7 (c 1.3, CH3OH); IR (KBr) ν 3330, 2928, 1272, 551 cm−1; 1H NMR (CDCl3) δ 0.88 (3H, t, J 7.0, CH3), 1.14–1.40 (16H, m, CH2), 1.50–1.58 (2H, m, CH2), 2.12–2.30 (1H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.60 (2H, m), 3.72–3.97 (2H, m); MS (EI) m/z 248, 250 (M+), 232 (M − H2O) [Calc. for C13H27ClO2 (250.8050): C, 62.26; H, 10.85. Found: C, 62.14; H, 10.67%].
)-1-Chlorotridecane-2,3-diol 6f..
A white solid (440 mg, 88%); mp 90–91 °C; [α]D
−9.7 (c 1.3, CH3OH); IR (KBr) ν 3330, 2928, 1272, 551 cm−1; 1H NMR (CDCl3) δ 0.88 (3H, t, J 7.0, CH3), 1.14–1.40 (16H, m, CH2), 1.50–1.58 (2H, m, CH2), 2.12–2.30 (1H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.60 (2H, m), 3.72–3.97 (2H, m); MS (EI) m/z 248, 250 (M+), 232 (M − H2O) [Calc. for C13H27ClO2 (250.8050): C, 62.26; H, 10.85. Found: C, 62.14; H, 10.67%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1-Chlorotetradecane-2,3-diol 6g..
A white solid: yield 454 mg, 86%); mp 89–90 °C; [α]D
−6.8 (c 1.0, CH3OH); IR (KBr) ν 3330, 2928, 1230, 563 cm−1; 1H NMR (CDCl3) δ 0.9 (3H, t, J 7.0, CH3), 1.15–1.40 (18H, m, CH2), 1.50–1.60 (2H, m, CH2), 2.12–2.30 (1H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.60 (2H, m), 3.60–3.98 (2H, m); MS (EI) m/z 262, 264 (M+), 246 (M − H2O) [Calc. for C14H29ClO2 (264.8316): C, 63.49; H, 11.04. Found: C, 63.31; H, 11.11%].
)-1-Chlorotetradecane-2,3-diol 6g..
A white solid: yield 454 mg, 86%); mp 89–90 °C; [α]D
−6.8 (c 1.0, CH3OH); IR (KBr) ν 3330, 2928, 1230, 563 cm−1; 1H NMR (CDCl3) δ 0.9 (3H, t, J 7.0, CH3), 1.15–1.40 (18H, m, CH2), 1.50–1.60 (2H, m, CH2), 2.12–2.30 (1H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.50–3.60 (2H, m), 3.60–3.98 (2H, m); MS (EI) m/z 262, 264 (M+), 246 (M − H2O) [Calc. for C14H29ClO2 (264.8316): C, 63.49; H, 11.04. Found: C, 63.31; H, 11.11%].
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1-Chloropentadecane-2,3-diol 6h..
A white solid (478 mg, 86%); mp 92–93 °C; [α]D
−7.0 (c 1.0, CH3OH); IR (KBr) ν 3329, 2927, 1230, 562 cm−1; 1H NMR (CDCl3) δ 0.92 (3H, t, J 7.0, CH3), 1.14–1.40 (20H, m, CH2), 1.52–1.62 (2H, m, CH2), 2.12–2.30 (1H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.53–3.62 (2H, m), 3.64–4.0 (2H, m); MS (EI) m/z 276, 278 (M+), 260 (M − H2O) [Calc. for C15H31ClO2 (278.8581): C, 64.61; H, 11.21. Found: C, 64.46; H, 11.42%].
)-1-Chloropentadecane-2,3-diol 6h..
A white solid (478 mg, 86%); mp 92–93 °C; [α]D
−7.0 (c 1.0, CH3OH); IR (KBr) ν 3329, 2927, 1230, 562 cm−1; 1H NMR (CDCl3) δ 0.92 (3H, t, J 7.0, CH3), 1.14–1.40 (20H, m, CH2), 1.52–1.62 (2H, m, CH2), 2.12–2.30 (1H, br s, OH), 2.60–2.70 (1H, br s, OH), 3.53–3.62 (2H, m), 3.64–4.0 (2H, m); MS (EI) m/z 276, 278 (M+), 260 (M − H2O) [Calc. for C15H31ClO2 (278.8581): C, 64.61; H, 11.21. Found: C, 64.46; H, 11.42%].
Compounds 6a–h can be easily transformed into the corresponding epoxy tosyl esters 4a–h upon treatment with K2CO3–MeOH and NaH–Tos-im. The typical procedure is as follows.
To a solution of 1.74 g of 6g (6.64 mmol) in methanol (10 ml) was added potassium carbonate (1.08 g, 7.82 mmol) and the reaction mixture was stirred at room temperature for 8 h. The reaction was quenched by addition of water (20 ml) and the mixture was extracted with ethyl acetate (10 ml × 3). The combined organic layer was dried over MgSO4 and concentrated under reduced pressure. The residue was treated with NaH (60%) (266 mg, 6.64 mmol) in anhydrous THF (20 ml) at 0 °C and the mixture was stirred at room temperature for 15 min. Tos-Im (1.45 g, 6.64 mmol) was added and the mixture was stirred for 1.5 h. The mixture was poured into ice–water and extracted with EtOAc. The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography (eluent: EtOAc–petroleum spirit 1∶6) to obtain compound 4g as a colorless solid (1.28 g, 50%); mp 53–56 °C; [α]D +8.4 (c 1, CHCl3).
The synthesis of insect pheromone (6Z,9S,10R)-9,10-epoxyhenicosadec-6-ene 9
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Hydroxy-10-(tosyloxy)icos-6-yne 7..
To a solution of hept-1-yne (300 mg, 3.6 mmol) in 5 ml of anhydrous THF was added a solution of n-BuLi (2.0 M; 1.8 ml, 3.6 mmol) in hexane at −78 °C. The resulting dark yellow solution was stirred for 30 min and then treated with boron trifluoride–diethyl ether (0.45 ml, 511 mg, 3.6 mmol) with syringe. After stirring of the mixture for another 30 min, a solution of 4g (500 mg, 1.3 mmol) in anhydrous THF (5 ml) was added and the mixture was stirred for 2 h at −78 °C. The reaction mixture was quenched by adding water, washed with aq. NH4Cl and extracted with diethyl ether. The organic layer was washed successively with water and brine, and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and the residue was purified by column chromatography to afford 7 (425 mg, 70%) as a colorless liquid, [α]D +6.4 (c 1, CHCl3) IR(neat) ν 3400, 2210 cm−1; 1H NMR (CDCl3): δ 0.89 (3H, t, J 6.0, CH3), 0.91 (3H, t, J 6.0, CH3), 1.10–1.70 (26H, m, CH2), 2.10–2.25 (2H, m, CH2), 2.27 (1H, br s, OH), 2.30–2.40 (2H, m, CH2), 2.45 (3H, s, CH3), 3.80 (1H, m, CH), 4.60–4.70 (1H, m, CH), 7.30 (2H, d, J 7.5, ArH), 7.80 (2H, d, J 7.5, ArH); MS (EI) m/z 479 (MH+) [Calc. for C28H46O4S (478.7284): C, 70.25; H, 9.69. Found: C, 70.56; H, 9.77%].
)-9-Hydroxy-10-(tosyloxy)icos-6-yne 7..
To a solution of hept-1-yne (300 mg, 3.6 mmol) in 5 ml of anhydrous THF was added a solution of n-BuLi (2.0 M; 1.8 ml, 3.6 mmol) in hexane at −78 °C. The resulting dark yellow solution was stirred for 30 min and then treated with boron trifluoride–diethyl ether (0.45 ml, 511 mg, 3.6 mmol) with syringe. After stirring of the mixture for another 30 min, a solution of 4g (500 mg, 1.3 mmol) in anhydrous THF (5 ml) was added and the mixture was stirred for 2 h at −78 °C. The reaction mixture was quenched by adding water, washed with aq. NH4Cl and extracted with diethyl ether. The organic layer was washed successively with water and brine, and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and the residue was purified by column chromatography to afford 7 (425 mg, 70%) as a colorless liquid, [α]D +6.4 (c 1, CHCl3) IR(neat) ν 3400, 2210 cm−1; 1H NMR (CDCl3): δ 0.89 (3H, t, J 6.0, CH3), 0.91 (3H, t, J 6.0, CH3), 1.10–1.70 (26H, m, CH2), 2.10–2.25 (2H, m, CH2), 2.27 (1H, br s, OH), 2.30–2.40 (2H, m, CH2), 2.45 (3H, s, CH3), 3.80 (1H, m, CH), 4.60–4.70 (1H, m, CH), 7.30 (2H, d, J 7.5, ArH), 7.80 (2H, d, J 7.5, ArH); MS (EI) m/z 479 (MH+) [Calc. for C28H46O4S (478.7284): C, 70.25; H, 9.69. Found: C, 70.56; H, 9.77%].
![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH); MS (EI) m/z 309 (MH+) [Calc. for C21H40O (308.5417): C, 81.75; H, 13.07. Found: C, 81.80; H, 13.12%].
CH); MS (EI) m/z 309 (MH+) [Calc. for C21H40O (308.5417): C, 81.75; H, 13.07. Found: C, 81.80; H, 13.12%].
Acknowledgements
We thank the National Natural Sciences Foundation of China for financial support.References
- T. Liljetors, M. Bengtsson and B. S. Hansson, J. Chem. Ecol., 1987, 13, 2023 Search PubMed; E. Hecker and A. Butenanot , in Techniques in Pheromone Research, ed. H. E. Hummel and T. A. Miller, Springer-Verlag, New York, 1984, ch. 1, pp. 1–44; Search PubMed; W. L. Roelofs , in Chemical Ecology: Odour Communication in Animals, ed. F. J. Ritter, Elsevier/North Holland Biomedical Press, New York, 1979, pp. 159–168; Search PubMed; W. L. Roelofs, A. Hill and R. Carde, J. Chem. Ecol., 1975, 1, 83 Search PubMed; J. A. Klun, O. L. Chapman, K. C. Mattes, P. W. Wojtkowski, M. Beroza and P. E. Sonnet, Science, 1973, 181, 661 Search PubMed.
- K. Mori , in Techniques in Pheromone Research, ed. H. E. Hummel and T. A. Miller, Springer-Verlag: New York, 1984, ch. 12, pp. 323–370; Search PubMed; T. W. Bell and J. Meinwald, J. Chem. Ecol., 1986, 12, 383 Search PubMed; J. Wunderer, K. Hansen, T. W. Bell, D. Schneider and J. Meinwald, J. Exp. Biol., 1986, 46, 11 Search PubMed; K. Hansen, Physiol. Entomol., 1984, 9, 9 Search PubMed; D. Schneider , in Comparative Physiology of Sensory Systems, ed. L. Bolis, R. D. Keynes and S. H. P. Maddrell, Cambridge University Press, London, 1984, p. 301. CAS.
- K. Mori, Chirality, 1998, 10, 578 CrossRef CAS.
- J. W. Wong, E. W. Underhill, S. L. McKenzie and M. D. Chisholm, J. Chem. Ecol., 1985, 11, 726 Search PubMed and references cited therein..
- J. S. Yadav and P. Radhakrishna, Tetrahedron, 1990, 46, 5825 CrossRef CAS and references cited therein..
- G. B. Payne, J. Org. Chem., 1962, 27, 3819 CrossRef CAS.
- T. W. Bell and J. A. Clacclo, Tetrahedron Lett., 1988, 29, 865 CrossRef CAS and references cited therein..
- J. S. Yadav, M. Y. Valli and A. R. Prasad, Tetrahedron, 1998, 54, 7551 CrossRef CAS and references cited therein..
- T. Soulie, T. Boyer and J. Y. Lallemand, Tetrahedron: Asymmetry, 1995, 6, 625 CrossRef.
- H. C. Kolb, M. S. VanNieuwenhze and K. B. Sharpless, Chem. Rev., 1994, 94, 2483 CrossRef CAS.
- Z.-B. Zhang, Z.-M. Wang, Y.-X. Wang, H.-Q. Liu, G.-X. Lei and M. Shi, Tetrahedron: Asymmetry, 1999, 10, 837 CrossRef CAS.
- G.-Q. Lin, Z.-X. Tan and Y.-W. Wu, Acta Chim. Sin., 1984, 42, 1178 Search PubMed.
- The ee was determined by Chiral HPLC (Chiralcel OD).
- C. Eisenberg and P. Knochel, J. Org. Chem., 1994, 59, 3746.
- T. Ebata and K. Mori, Agric. Biol. Chem., 1989, 53, 801 Search PubMed; T. W. Bell and J. Ciaccio, J. Org. Chem., 1993, 58, 5153 CrossRef CAS.
- K. Mori and T. Ebata, Tetrahedron, 1986, 42, 3471 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2000 |
