Lipase promoted asymmetric trans-esterification of 4-alkyl-, 3-alkyl- and 3,4-dialkyloxetan-2-ones with ring-opening
Naoko
Sakai
,
Satoru
Ageishi
,
Hiroshi
Isobe
,
Yoshiyuki
Hayashi
and
Yukio
Yamamoto
*
Graduate School of Human and Environmental Studies, Kyoto University, Sakyo-ku, Yoshida, Kyoto, 606-8501, Japan
First published on 12th January 2000
Abstract
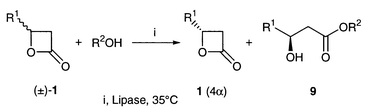
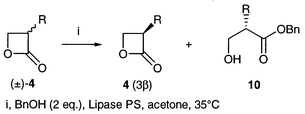
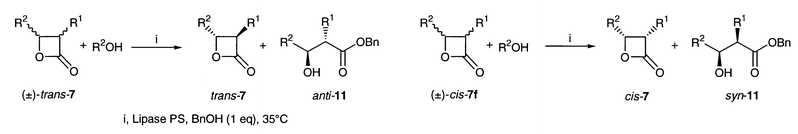
Kinetic resolution of (±)-4-substituted [(±)-1], 3-substituted [(±)-4] and 3,4-disubstituted oxetan-2-ones [(±)-7] was effected by the action of lipases in organic solvents. The substrates (±)-1, (±)-4 and (±)-7 were prepared by [2 + 2] cycloaddition of aldehydes with ketene, intramolecular substitution of 3-bromoalkanoic acids and the Adams cyclization of anti- and syn-3-hydroxyalkanoic acids, respectively. Lipase PS exhibited good activity towards all the oxetanones and was employed for the resolution experiments except with (±)-4-methyloxetan-2-one (±)-1a for which PPL was used. The stereoselectivity was satisfactory for obtaining oxetan-2-ones of high ee’s except for a few cases. The configuration of new compounds was established by chemical correlation and CD spectroscopy.
Optically active oxetan-2-ones, β-lactones, have attracted much attention because they are found in many biologically active natural products
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 1,2 and have also been utilized as monomers in the preparation of biodegradable poly(hydroxyalkanoates).3,4 Moreover, they can be employed as chiral building blocks having two centres that are reactive towards nucleophiles.1 Optically active 4-substituted oxetan-2-ones have been synthesized from optically active 3-halo- and 3-hydroxyalkanoic acids. Asymmetric syntheses of these compounds have also been reported, including the stereoselective synthesis of 3,4-dialkyloxetan-2-one from optically active 3-methyloxetan-2-one,5 the asymmetric hydrogenation of diketene
1,2 and have also been utilized as monomers in the preparation of biodegradable poly(hydroxyalkanoates).3,4 Moreover, they can be employed as chiral building blocks having two centres that are reactive towards nucleophiles.1 Optically active 4-substituted oxetan-2-ones have been synthesized from optically active 3-halo- and 3-hydroxyalkanoic acids. Asymmetric syntheses of these compounds have also been reported, including the stereoselective synthesis of 3,4-dialkyloxetan-2-one from optically active 3-methyloxetan-2-one,5 the asymmetric hydrogenation of diketene![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 6 and the asymmetric cycloaddition of ketene and trichloroacetaldehyde.7 These methods have afforded oxetan-2-ones in high ee’s but their structure is restricted to 4-alkyl-3-methyl-, 4-methyl- and 4-trichloromethyloxetan-2-one, respectively. Recently, asymmetric cycloadditions were reported in which various aldehydes reacted with ketene
6 and the asymmetric cycloaddition of ketene and trichloroacetaldehyde.7 These methods have afforded oxetan-2-ones in high ee’s but their structure is restricted to 4-alkyl-3-methyl-, 4-methyl- and 4-trichloromethyloxetan-2-one, respectively. Recently, asymmetric cycloadditions were reported in which various aldehydes reacted with ketene![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 8 or trimethylsilylketene
8 or trimethylsilylketene![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 9a in the presence of chiral Lewis acids.9b However, the selectivity was moderate or low. In this context, we planned to develop an enzymatic procedure that would yield oxetan-2-ones in high ee’s.
9a in the presence of chiral Lewis acids.9b However, the selectivity was moderate or low. In this context, we planned to develop an enzymatic procedure that would yield oxetan-2-ones in high ee’s.
Lipase has been widely used for asymmetric synthesis and optical resolution.10 Asymmetric hydrolysis of lactones which are 5-membered rings or bigger![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 11 and asymmetric lactonization of hydroxy esters
11 and asymmetric lactonization of hydroxy esters![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 12 have been reported. Diketene (4-methyleneoxetan-2-one), having a similar structure, catalyzed by lipase has been employed for the resolution of alcohols with high stereoselectivity.13 We have previously reported the facile optical resolution of 4-alkyloxetan-2-ones with lipase and alcohols in organic solvents. The reaction is promoted by releasing the strain in the 4-membered ring.14 Recently, 4-alkyl-3-methyleneoxetan-2-ones
12 have been reported. Diketene (4-methyleneoxetan-2-one), having a similar structure, catalyzed by lipase has been employed for the resolution of alcohols with high stereoselectivity.13 We have previously reported the facile optical resolution of 4-alkyloxetan-2-ones with lipase and alcohols in organic solvents. The reaction is promoted by releasing the strain in the 4-membered ring.14 Recently, 4-alkyl-3-methyleneoxetan-2-ones![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 15 and 4-haloalkyloxetan-2-ones
15 and 4-haloalkyloxetan-2-ones![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 16 were resolved by this procedure. Here, we describe the lipase promoted asymmetric trans-esterification of (±)-4-substituted [(±)-1], 3-substituted [(±)-4] and 3,4-disubstituted oxetan-2-ones [(±)-7] and aim to clarify the scope and limitation of this method.
16 were resolved by this procedure. Here, we describe the lipase promoted asymmetric trans-esterification of (±)-4-substituted [(±)-1], 3-substituted [(±)-4] and 3,4-disubstituted oxetan-2-ones [(±)-7] and aim to clarify the scope and limitation of this method.
The synthesis of racemic oxetan-2-ones is summarized in Scheme 1. 4-Alkyloxetan-2-ones (±)-1 were prepared by the [2 + 2] cycloaddition of aldehydes with ketene, catalyzed by boron trifluoride (except for (±)-1a which was formed by hydrogenation of diketene).17 By the action of aqueous sodium hydroxide, 3-alkyloxetan-2-ones (±)-4 were prepared from 3-bromoalkanoic acids (±)-3 which were derived from unsaturated acids 2.18 Stereoselective aldol reactions gave (±)-anti-6a–e![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 19 and (±)-syn-6f
19 and (±)-syn-6f![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 20 from which (±)-trans-7a–e and (±)-cis-7f were afforded by Adams’ cyclization.21
20 from which (±)-trans-7a–e and (±)-cis-7f were afforded by Adams’ cyclization.21
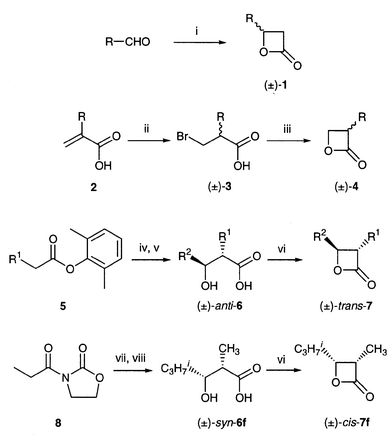 | ||
Scheme 1
Reagents and conditions: i, CH2![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) C C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O, BF3; ii, HBr; iii, NaOH, iv, LDA, R2CHO, −78 °C; v, KOH; vi, TsCl, pyridine, 0 °C; vii, (C4H9n)2BOTf, (C2H5)3N, C3H7iCHO, −78 °C; viii, H2O2, LiOH. O, BF3; ii, HBr; iii, NaOH, iv, LDA, R2CHO, −78 °C; v, KOH; vi, TsCl, pyridine, 0 °C; vii, (C4H9n)2BOTf, (C2H5)3N, C3H7iCHO, −78 °C; viii, H2O2, LiOH.
| ||
4-Methyloxetan-2-one 1a was chosen for the initial optimization study and reacted with ethanol, butan-1-ol and benzyl alcohol to give alkyl 3-hydroxybutanoates in the presence of porcine pancreas lipase (PPL) and Lipase PS (Pseudomonas sp. lipase) in various organic solvents. The products are those expected from ordinary lipase promoted trans-esterification and are generated by acyl fission of 1a, which is observed in basic hydrolysis of oxetan-2-ones (while acidic hydrolysis proceeds by alkyl fission).22 By using the E value calculated from the ee of the remaining oxetanone (ees) and the reaction conversion [eqn. (1)],23 we examined various combinations of lipases, alcohols and solvents. It turned out that the reaction proceeded with very high stereoselectivity when using PPL, benzyl alcohol and acetone. Based on this preliminary experiment, a preparative resolution was carried out to obtain (+)-1a (96% ee) and benzyl 3-hydroxybutanoate (+)-9a (85% ee) in good yields (Table 1, entry 1). From these values we calculated an E value of 39 [eqn. (2)] which is a more reliable method than eqn. (1).24
| E = [ln(1 − c)(1 − ees)]/[ln(1 − c)(1 + ees)] | (1) |
| E = [ln(1 − ees)(ees + eep)]/[ln(1 + ees)(ees + eep)] | (2) |
| Conversion = ln[ees/(ees + eep)] | (3) |
|
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxetanone 1 | Ester 9 | |||||||||||||
| Entry | Substrate | R1 | R2 | Lipase | Solvent | t/h | Conv. (%)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
Yield (%) | [α]25D/10−1 deg cm2 g−1 (c, CHCl3) | Ee (%) | Yield (%) | [α]25D/10−1 deg cm2 g−1 (c, CHCl3) | Ee (%)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
| a Calculated from eqns. (2) and (3). b Determined by 1H NMR using Eu(hfc)3. c Determined by method A. Finepak SIL; retention time [amide from (R)-1a] 23.6 min, [amide from (S)-1a] 25.5 min. The R configuration was assigned by optical rotation (ref. 6). d Determined by method B: methyl ester 13b [α]25D −3.5 (c 2.5, EtOH) [(S)-isomer, [α]25D +4.6 (c 1.0, EtOH), 98% ee] (ref.26); methyl ester 13c [α]25D −25.3 (c 2.2, EtOH) [(S)-isomer, [α]25D −23.5 (c 5.0, EtOH), 80% ee] (ref. 27); methyl ester 13d [α]25D −19.1 (c 1.6, CHCl3) [(S)-isomer, [α]25D −27.1 (c 1.5, CHCl3), >99% ee] (ref. 28); acid 14f [α]25D −11.3 (c 1.2, CHCl3) [(R)-isomer, [α]25D −16.2 (c 1.0, CHCl3), >99% ee] (ref. 29). | ||||||||||||||
| 1 | 1a | CH3 | Bn | PPL | Acetone | 144 | 54 | 39 | 36 | +28.0 (4.3) | 96![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) c c |
51 | +25.7 (1.1) | 85 |
| 2 | 1b | C3H7n | Bn | PS | Acetone | 96 | 52 | 12 | 42 | +32.8 (1.1) | 75![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) d d |
45 | +14.1 (1.1) | 69 |
| 3 | 1c | C3H7i | Bn | PS | Acetone | 288 | 51 | 70 | 41 | +22.8 (1.1) | 95![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) d d |
43 | +27.4 (1.0) | 90 |
| 4 | 1d | C4H9n | Bn | PS | Acetone | 504 | 47 | 20 | 46 | +26.0 (1.5) | 71![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) d d |
36 | +16.1 (1.5) | 81 |
| 5 | 1d | C4H9n | C4H9n | PS | (C3H7i)2O | 44 | 57 | 16 | 30 | +32.9 (1.5) | 90![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) d d |
40 | +15.2 (1.4) | 68 |
| 6 | 1e | C4H9t | C4H9n | PS | (C3H7i)2O | 200 | <1 | — | — | — | — | — | — | — |
| 7 | 1f | C11H23n | Bn | PS | Acetone | 216 | 48 | 16 | 27 | +14.7 (1.2) | 70![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) d d |
25 | +9.7 (1.1) | 77 |
| 8 | 1f | C11H23n | C4H9n | PS | (C3H7i)2O | 14 | 42 | 16 | 52 | +12.0 (1.2) | 57![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) d d |
35 | +14.1 (1.1) | 80 |
The reactions of 4-alkyloxetan-2-ones (±)-1 having primary alkyl groups, including those with long chains, and isopropyl groups were very sluggish with PPL but they were effectively catalyzed by Lipase PS to give the products (+)-1 and (+)-9 in high ee (Table 1). The best selectivity (E = 70) was attained with 1c, which has a secondary alkyl group, whilst moderate E’s were obtained with oxetanones which have primary alkyl groups. The reaction of (±)-1a (R = CH3) was catalyzed effectively by Lipase PS but the selectivity was low (E = 7). The tert-butyl derivative (±)-1e did not react under any conditions (Table 1, entry 6). The configurations of the remaining oxetanones (+)-1 and esters (+)-9 are S and R, respectively, except for 1c and 9c. This difference is due to the R,S-nomenclature and the spatial orientation at the stereogenic centre is the same. Because the R,S-notation could lead to serious confusion, the structure is given in Table 1 and we have used the terminology employed in steroid chemistry to identify the stereochemistry, i.e. α (below the plane of the paper) and β (above the plane). Accordingly, the 4-alkyloxetan-2-ones 1 have an α-4-alkyl group. The long reaction periods could be reduced by employing butan-1-ol and diisopropyl ether as the nucleophile and solvent, respectively, without lowering the stereoselectivity (Table 1, entries 5 and 8). In the case of the isopropyl derivative (±)-1c, the reaction periods were almost the same with benzyl alcohol–acetone and butan-1-ol–diisopropyl ether.
Based on the results of the 4-alkyloxetan-2-ones (±)-1, the asymmetric trans-esterification of 3-alkyloxetan-2-ones (±)-4 was effected but the results were not as good (Table 2). In addition to ester 10, dimers and trimers were found when an equimolar amount of alcohol was employed. They were derived from acylation of 10 with oxetanone 4 and their formation was suppressed by using two equivalents of alcohol. The methyl derivative (±)-4a and the butyl derivative (±)-4c were resolved with moderate and low selectivity, respectively (Table 2, entries 1 and 3); they have the 3R configuration and a 3β-substituent. Oxetanone 4b, which has an isopropyl group, did not react at all.
|
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxetanone 4 | Ester 10 | ||||||||||
| Entry | Substrate | R | t/h | Conv. (%)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
Yield (%) | [α]25D/10−1 deg cm2 g−1 (c, CHCl3) | Ee (%) | Yield (%) | [α]25D/10−1 deg cm2 g−1 (c, CHCl3) | Ee (%) |
| a Calculated from eqns. (2) and (3). b Based on the reported maximum rotation [(R)-isomer, [α]25D +10.5 (c 1.0, CHCl3), >99% ee] (ref.30). c Compared the rotation [[α]25D +9.1 (c 10.3, EtOH)] of hydroxy acid derived from 10a by hydrogenolysis with the reported maximum rotation [(S)-isomer, [α]25D +12.7 (c 12.5, EtOH), >99% ee] (ref.31). d Chiral GC, ASTEC CHIRALDEX B-PH; retention time [(3R)-4a] 20.6 min, [(3S)-4a] 21.0 min. e Determined by method C. | |||||||||||
| 1 | 4a | CH3 | 9 | 49 | 13 | 38 | +7.4 (9.4) | 70![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
24 | +13.2 (10.0) | 72![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) c c |
| 2 | 4b | C3H7i | 192 | <1 | — | — | — | — | — | — | — |
| 3 | 4c | C4H9n | 204 | 49 | 3 | 45 | −8.6 (9.3) | 32![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) d d |
32 | +1.8 (8.8) | 36![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) e e |
The reactions of 3,4-dialkyloxetan-2-ones (±)-7 were slower than those of both 4-alkyl- and 3-alkyloxetanones (Table 3). Oxetanones (±)-trans-7c, having tert-butyl group, and (±)-trans-7e, having both 3-propyl and 4-isopropyl groups, did not react (Table 3, entries 3 and 5). The trans-esterification proceeded with a considerably high selectivity in the cases where one of the two substituents was a methyl group. Both the trans and cis forms of 3,4-dialkyloxetan-2-ones 7 have a 4α-substituent regardless of the orientation of the 3-substituents. This configuration is in accord with that expected from the more selective reaction of 4-alkyloxetan-2-ones 1 and the less selective reaction of 3-alkyloxetan-2-ones 4. It can be concluded that the stereochemical course of the reaction is mainly directed by the 4-substituent.
|
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxetanone 7 | Ester 11 | |||||||||||||
| Entry | Substrate | R1 | R2 | t/day | Conv. (%)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
Yield (%) | [α]25D/10−1 deg cm2 g−1 (c, CHCl3) | Ee (%) | Config. | Yield (%) | [α]25D/10−1 deg cm2 g−1 (c, CHCl3) | Ee (%) | Config. |
a
Calculated from eqns. (2) and (3).
b
Determined by method D. DAICEL CHIRALPAK AD.
c
Determined by method D. DAICEL CHIRALPAK OJ.
d
Determined by Chiral HPLC, CHIRALPAK AD; retention time [(2S,3S)-11a] 19.9 min, [(2R,3R)-11a] 18.8 min.
e
Determined by Chiral HPLC, CHIRALPAK OJ; retention time [(2S,3S)-11b] 20.5 min, [(2R,3R)-11b] 18.5 min.
f
Determined by Chiral HPLC, CHIRALPAK AD; retention time [(2S,3S)-11d] 12.9 min, [(2R,3R)-11d] 6.2 min.
g
51% ee.
h
Chiral GC, ASTEC CHIRALDEX B-PH; retention time [(3S,4R)-7f![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ] 12.5 min, [(3R,4S)-7f ] 12.5 min, [(3R,4S)-7f![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ] 13.6 min.
j
Determined by method E. ] 13.6 min.
j
Determined by method E.
|
||||||||||||||
| 1 | trans-7a | CH3 | C3H7n | 11 | 51 | 50 | 50 | +41.3 (1.1) | 92![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
3R,4R (3β,4α) | 34 | +1.3 (c 1.1) | 87![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) d d |
2S,3S |
| 2 | trans-7b | CH3 | C3H7i | 15 | 46 | 26 | 37 | +34.6 (1.2) | 72![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) c c |
3R,4R (3β,4α) | 41 | +15.2 (1.2) | 85![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) e e |
2S,3S |
| 3 | trans-7c | CH3 | C4H9t | 14 | <1 | — | — | — | — | — | — | — | — | — |
| 4 | trans-7d | C3H7n | CH3 | 21 | 45 | >99 | 16 | +11.4 (0.92) | 79![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
3R,4R (3β,4α) | 24 | +4.4 (0.92) | 98![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) f f |
2S,3S |
| 5 | trans-7e | C3H7n | C3H7i | 14 | <1 | — | — | — | — | — | — | — | — | — |
| 6 | cis-7f | CH3 | C3H7i | 20 | 50 | 32 | 13 | −1.3 (2.3)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) g g |
85![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) h h |
3S,4R (3α,4α) | 39 | +5.8 (0.40) | 84![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) j j |
2R,3S |
The methods used to directly determine the ee and configuration of the compounds, 4a, 4c, cis-7f, 9a–d, 9f, 10a and anti-11a, 11b, are given as footnotes to the relevant Table. The methods of determining the ee’s of the others are summarized in Scheme 2. The ee of oxetanone 1a was assessed by HPLC after converting to a diastereomeric mixture of amide 12 (method A). The other 4-alkyloxetan-2-ones were converted to the corresponding acids or esters and the specific rotations were measured (method B). Ester 10c was cyclized to oxetanone 4 and chiral GC analysis was performed (method C). After converting trans-7a, 7b and 7d to the corresponding benzyl esters, their ee’s were assessed by chiral HPLC (method D). Ester syn-11f was cyclized to cis-7f on which chiral GC was carried out (method E).
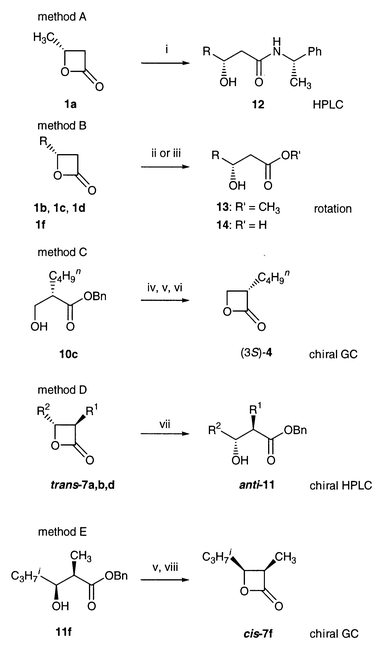 | ||
| Scheme 2 Determination of ee; reagents and conditions: i, (S)-1-phenylethylamine, 130 °C; ii, CH3OH, reflux; iii, NaOH, rt; iv, MsCl, (C2H5)3N, 0 °C; v, H2/Pd–C, vi, NaOH, vii, BnOH, NaH; viii, TsCl, pyridine, 0 °C. | ||
In order to determine the configuration of disubstituted oxetanone cis-7f, it was independently synthesized by Evans’ asymmetric aldol reaction followed by Adams’ cyclization (Scheme 3). On chiral GC analysis, the retention time of the major isomer derived from the asymmetric synthesis coincided with that from the present enzymatic reaction, the 3S,4R configuration (3α-methyl and 4α-isopropyl) having been assigned to the latter. Epimerization of (3S,4R)-cis-7f at position 3 with LDA gave four base-line separated peaks on the chiral GC. The major peak observed is assigned to (3R,4R)-trans-7b (3β-methyl and 4α-isopropyl) whose retention time coincided with that from the present enzymatic reaction of (±)-trans-7b. The 3β,4α orientation of the alkyl groups of the other 3,4-dialkyloxetan-2-ones trans-7a,d was confirmed by the comparison of their CD spectra (Table 4). The 3R configuration of 4c (R = Bun) was also established by this method, and by comparing it with (3R)-4a (R = Me). All the compounds showed a negative maximum at 214–217 nm.
![Reagents and conditions: i, (C4H9n)2BOTf, (C2H5)3N, 0 °C, then C3H7iCHO, −78 °C; ii, TsCl, pyridine; iv, LDA then CH3CO2H; v, Chiral GC ASTEC CHIRALDEX B-PH; rt [(3S,4R)-cis-7f ] 12.5 min (major), rt [(3R,4S)-cis-7f ] 13.6 min (minor), rt [(3R,4R)-trans-7f ] 6.9 min (major), rt [(3S,4S)-trans-7f ] 7.1 min (minor).](/image/article/2000/P1/a907220i/a907220i-s3.gif) | ||
Scheme 3
Reagents and conditions: i, (C4H9n)2BOTf, (C2H5)3N, 0 °C, then C3H7iCHO, −78 °C; ii, TsCl, pyridine; iv, LDA then CH3CO2H; v, Chiral GC ASTEC CHIRALDEX B-PH; rt [(3S,4R)-cis-7f![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ] 12.5 min (major), rt [(3R,4S)-cis-7f ] 12.5 min (major), rt [(3R,4S)-cis-7f![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ] 13.6 min (minor), rt [(3R,4R)-trans-7f ] 13.6 min (minor), rt [(3R,4R)-trans-7f![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ] 6.9 min (major), rt [(3S,4S)-trans-7f ] 6.9 min (major), rt [(3S,4S)-trans-7f![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ] 7.1 min (minor). ] 7.1 min (minor).
| ||
In conclusion, this report describes a methodology for the preparation of optically active oxetan-2-ones of moderate to high ee’s, and the substrate structure which permits the lipase promoted trans-esterification.
Moreover, the orientation of the ring substituents, which directs the stereochemistry of the reaction has been identified. The resolution of 4-alkyloxetan-2-ones 1 is especially efficient in the enzymatic preparation methods discussed here. Oxetan-2-ones 1 and 3-hydroxyalkanoates, readily derived from 1, are useful chiral building blocks. In addition, it is very interesting that natural products containing the oxetan-2-one moiety are potent inhibitors of some lipases, including PPL.25 The results from this work and further application of these methods to oxetan-2-ones with long alkyl substituents should help to identify the catalysis and inhibition mechanisms. The reasons for the inhibition towards PPL hydrolysis and trans-esterification by the oxetan-2-ones resolved by Lipase PS are currently under investigation.
Experimental
1H NMR and 13C NMR spectra were recorded with a JEOL-JNM-EX-270 spectrometer (at 270 MHz for 1H, 68 MHz for 13C). J Values are given in Hz. IR spectra were recorded with a SHIMADZU FTIR-8600PC spectrophotometer. Optical rotation was measured with a JASCO DIP-1000 polarimeter (with a 10 cm cell). [α] values are given as 10−1 deg cm2 g−1. HPLC analyses were run on a JASCO 880-PU chromatographic system with an 875-UV detector (220 nm) and a silica gel column (FINPAK SIL, 4 mm × 25 cm). Chiral HPLC analyses were run with DAICEL CHIRALPAK AD and OJ. Chiral GC-mass analyses were done on a Shimadzu GC-17A with ASTEC CHIRALDEX B-PH. High resolution mass spectra (HRMS) were recorded with a JEOL JMS DX-300 spectrometer. CD spectra were measured with a JASCO J-720 CD spectrometer (with a 0.1 cm cell). The elemental analyses were performed by Kyoto University elemental analysis center.(±)-4-Methyloxetan-2-one (±)-1a
The desired oxetan-2-one (±)-1a (86%) was yielded by hydrogenation of diketene.17General method for the preparation of (±)-4-alkyloxetan-2-ones
Ketene gas was passed into a solution of BF3·Et2O (0.1 eq.) in dry ether (50 cm3) at 0 °C for 5 min. Then, aldehyde (1.0 eq.) in dry ether (50 cm3) was added dropwise over 3 h to this solution at 10 °C during which the introduction of ketene gas was continued. After adding 50% aq. NaOH (2 cm3), the mixture was washed with 10% aq. citric acid (20 cm3 × 2) and saturated NaCl (20 cm3). Then the organic layer was dried over MgSO4, filtered and evaporated. The residue was purified by distillation or silica gel flash chromatography.![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H), 3.32 (dd, J 6.2, 11.1, 1H, COCH
H), 3.32 (dd, J 6.2, 11.1, 1H, COCH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H), 4.2–4.3 (m, 1H, CHC4H9t); δC (CDCl3) 24.0, 32.8, 38.2, 77.9, 168.3.
H), 4.2–4.3 (m, 1H, CHC4H9t); δC (CDCl3) 24.0, 32.8, 38.2, 77.9, 168.3.
General method for preparation of 3-alkyloxetan-2-ones
To a solution of HBr in CH3CO2H (25%, 1.1 eq.), acid 2 was added dropwise at 0 °C. After stirring overnight, the solution was evaporated. The residue was purified by distillation to give bromo acid (±)-3. A solution of NaOH (1 M, 1.0 eq.) was added to (±)-3 slowly. After adding CHCl3, the mixture was stirred vigorously. The organic layer was separated and CHCl3 was added to the aqueous layer. The mixture was stirred for 2 h and the organic layer was separated. The combined organic extracts were dried over MgSO4, filtered and evaporated. The residue was purified by distillation or silica gel flash chromatography.![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H), 4.31 (dd, J 5.4, 6.2, 1H, OCHCH); δC(CDCl3) 19.6, 20.2, 27.8, 58.9, 63.2, 171.6; IR (neat): 1820 cm−1.
H), 4.31 (dd, J 5.4, 6.2, 1H, OCHCH); δC(CDCl3) 19.6, 20.2, 27.8, 58.9, 63.2, 171.6; IR (neat): 1820 cm−1.
General method for preparation of trans- and cis-3,4-disubstituted oxetan-2-ones
The desired products (±)-trans-7a–e and (±)-cis-7f were prepared according to Heathcock![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 19 and Evans
19 and Evans![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 20 and their co-workers respectively, followed by Adams’ cyclization.21
20 and their co-workers respectively, followed by Adams’ cyclization.21
General method for asymmetric trans-esterification of oxetan-2-ones
A mixture of oxetan-2-one (58 mmol), alcohol (46 mmol), lipase (5.0 g) and solvent (50 cm3) was stirred at 35 °C during which the reaction conversion was assessed by 1H NMR. When the conversion reached ca. 50%, the lipase was filtered off and washed with ether (20 cm3) and the combined filtrate and washings were evaporated. Oxetan-2-one and ester were separated from the residue by fractional distillation or silica gel column chromatography.![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H), 3.57 (dd, J 5.9, 16, 1H, CH
H), 3.57 (dd, J 5.9, 16, 1H, CH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H), 4.6–4.8 (m, 1H, CHCH3); δC(CDCl3) 20.5, 44.2, 67.8, 168.2.
H), 4.6–4.8 (m, 1H, CHCH3); δC(CDCl3) 20.5, 44.2, 67.8, 168.2.
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H), 3.50 (dd, J 5.9, 16, 1H, CH
H), 3.50 (dd, J 5.9, 16, 1H, CH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H), 4.5–4.6 (m, 1H, CHC3H7n); δC(CDCl3) 13.5, 18.2, 36.6, 42.8, 71.1, 168.3.
H), 4.5–4.6 (m, 1H, CHC3H7n); δC(CDCl3) 13.5, 18.2, 36.6, 42.8, 71.1, 168.3.
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H), 3.41 (dd, J 5.7, 16, 1H, CH
H), 3.41 (dd, J 5.7, 16, 1H, CH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H), 4.2–4.3 (m, 1H, CHC3H7i); δC (CDCl3) 16.7, 17.6, 32.4, 40.8, 75.7, 168.3.
H), 4.2–4.3 (m, 1H, CHC3H7i); δC (CDCl3) 16.7, 17.6, 32.4, 40.8, 75.7, 168.3.
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) HCO), 3.51 (dd, J 5.7, 11.9, 1H, CH
HCO), 3.51 (dd, J 5.7, 11.9, 1H, CH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) HCO), 4.5–4.6 (m, 1H, OCHCH2); δC (CDCl3) 13.8, 22.2, 27.0, 34.3, 42.9, 71.3, 168.3.
HCO), 4.5–4.6 (m, 1H, OCHCH2); δC (CDCl3) 13.8, 22.2, 27.0, 34.3, 42.9, 71.3, 168.3.
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) HCO), 3.51 (dd, J 6.8, 11.9, 1H, CH
HCO), 3.51 (dd, J 6.8, 11.9, 1H, CH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) HCO), 4.5–4.6 (m, 1H, OCH); δC(CDCl3) 14.1, 22.6, 24.9, 29.1, 29.3, 29.36, 29.42, 29.5, 31.8, 34.6, 42.8, 71.3, 168.3.
HCO), 4.5–4.6 (m, 1H, OCH); δC(CDCl3) 14.1, 22.6, 24.9, 29.1, 29.3, 29.36, 29.42, 29.5, 31.8, 34.6, 42.8, 71.3, 168.3.
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H), 4.40 (dd, J 6.0, 6.0, 1H, OCH
H), 4.40 (dd, J 6.0, 6.0, 1H, OCH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H); δC(CDCl3) 12.9, 46.7, 66.4, 172.3.
H); δC(CDCl3) 12.9, 46.7, 66.4, 172.3.
Acknowledgements
We thank Amano Pharmaceutical Co. Ltd. for the gift of Lipase PS.References
- Review: A. Pommier and J. M. Pons, Synthesis, 1993, 41.
- S. Hanessian, A. Tehim and P. Chen, J. Org. Chem., 1993, 58, 7768 CrossRef CAS and references cited therein..
- Review: H. M. Müller and D. Seebach, Angew. Chem., Int. Ed. Engl., 1993, 32, 477 CrossRef review: I. Collins, J. Chem. Soc., Perkin Trans. 1, 1999, 1377 RSC.
- J. E. Kemnitzer, S. P. McCarthy and R. A. Gross, Macromolecules, 1993, 26, 6143 CrossRef CAS and references cited therein; J. E. Kemnitzer, S. P. McCarthy and R. A. Gross, Macromolecules, 1993, 26, 1221 CrossRef CAS; Y. Hori, M. Suzuki, A. Yamaguchi and T. Nishishita, Macromolecules, 1993, 26, 5533 CrossRef CAS.
- A. Griesbeck and D. Seebach, Helv. Chem. Acta, 1987, 70, 1320 Search PubMed; S. Cammas, I. Renard, K. Boutault and P. Guèrin, Tetrahedron: Asymmetry, 1993, 4, 1925 CrossRef CAS; G. Capozzi, S. Roelens and S. Talami, J. Org. Chem., 1993, 58, 7932 CrossRef CAS.
- T. Ohta, T. Miyake and H. Takaya, J. Chem. Soc., Chem. Commun., 1992, 1725 RSC; T. Ohta, T. Miyake, N. Seido, H. Kumobayashi and H. Takaya, J. Org. Chem., 1995, 60, 357 CrossRef CAS.
- H. Wynberg and E. G. J. Staring, J. Am. Chem. Soc., 1982, 104, 166 CrossRef CAS; P. E. F. Ketelaar, E. G. J. Starling and H. Wynberg, Tetrahedron Lett., 1985, 26, 4665 CrossRef CAS.
- Y. Tamai, H. Yoshiwara, M. Someya, J. Fukumoto and S. Miyano, J. Chem. Soc., Chem. Commun., 1994, 2281 RSC; Y. Tamai, M. Someya, J. Fukumoto and S. Miyano, J. Chem. Soc., Perkin Trans. 1, 1994, 1549 RSC.
- (a) B. W. Dymock, P. J. Kocienski and J.-M. Pons, Synthesis, 1998, 1655 CrossRef CAS; (b) An asymmetric synthesis of oxetan-2-ones of high ee’s was reported very recently: S. G. Nelson , T. J. Peelen and Z. Wan , J. Am. Chem. Soc., 1999, 121, 9742. Search PubMed.
- Reviews: W. Boland, C. Frößl and M. Lorenz, Synthesis, 1991, 1049 CrossRef CAS; K. Faber and S. Riva, Synthesis, 1992, 895 CrossRef CAS; E. Santaniello, P. Ferraboschi, P. Grisenti and A. Manzocchi, Chem. Rev., 1992, 92, 1071 CrossRef CAS; J. Otera, Chem. Rev., 1993, 93, 1449 CrossRef CAS.
- E. Fouque and G. Rousseau, Synthesis, 1989, 661 CrossRef CAS; L. Blanco, E. Guibé-Jampel and G. Rousseau, Tetrahedron Lett., 1988, 29, 1915 CrossRef; E. Guibé-Jampel, G. Rousseau and L. Blanco, Tetrahedron Lett., 1989, 30, 67 CrossRef CAS.
- H. Yamada, T. Sugai, H. Ohta and S. Yoshikawa, Agric. Biol. Chem., 1990, 54, 1579 Search PubMed; H. Yamada, S. Ohsawa, T. Sugai, H. Ohta and S. Yoshikawa, Chem. Lett., 1989, 1775 CAS; E. Fukusaki, S. Senda, Y. Nakazono and T. Omata, Tetrahedron, 1991, 47, 6223 CrossRef CAS; A. L. Gutman and T. Bravdo, J. Org. Chem., 1989, 54, 4263 CrossRef CAS.
- K. Suginaka, Y. Hayashi and Y. Yukio, Tetrahedron: Asymmetry, 1996, 7, 1153 CrossRef CAS; N. Öhner, M. Martinelle, A. Mattson, T. Norin and K. Hult, Biotechnol. Lett., 1992, 14, 263.
- Y. Koichi, K. Suginaka and Y. Yamamoto, J. Chem. Soc., Perkin Trans. 1, 1995, 1645 RSC.
- W. Adam, P. Groer and C. R. Saha-Möller, Tetrahedron: Asymmetry, 1997, 8, 833 CrossRef.
- T. Ito, M. Shimizu and T. Fujisawa, Tetrahedron, 1998, 54, 5523 CrossRef CAS.
- J. R. Clemens, Chem. Rev., 1986, 86, 241 CrossRef.
- L. Brikofer, A. Ritter and J. Schramn, Chem. Ber., 1962, 95, 426 Search PubMed.
- C. H. Heathcock, M. C. Pirrung, S. H. Montgomery and J. Lampe, Tetrahedron, 1981, 37, 4087 CrossRef CAS.
- D. A. Evans, J. Bartroli and T. L. Shih, J. Am. Chem. Soc., 1981, 103, 2127 CrossRef CAS.
- W. Adam, J. Baeza and J. Liu, J. Am. Chem. Soc., 1972, 22, 2000 CrossRef.
- S. E. Ramer, R. N. Moore and J. C. Vederas, Can. J. Chem., 1986, 64, 706 CAS.
- S. C. Chen, Y. Fujimoto, G. Girdaukas and J. C. Sih, J. Am. Chem. Soc., 1982, 104, 7294 CrossRef CAS.
- J. L. L. Rakels, A. J. J. Straathof and J. J. Heijnen, Enzyme Microb. Technol., 1993, 15, 1051 CrossRef CAS.
- P. Hadváry, H. Lengsfeld and H. Wolfer, Biochem. J., 1988, 256, 357 CAS; B. Borgström, Biochem. Biophys. Acta, 1988, 962, 308 Search PubMed reviews: R. D. Schmid and R. Verger, Angew. Chem., Int. Ed., 1998, 37, 1608 CrossRef.
- F. D. Taber, B. P. Deker and J. L. Silverberg, J. Org. Chem., 1992, 57, 5990 CrossRef.
- A. Tai, T. Harada, Y. Hiraki and S. Murakami, Bull. Chem. Soc. Jpn., 1983, 56, 1414 CAS.
- M. Utaka, H. Watabu, H. Higashi, T. Sakai, S. Tsuboi and S. Torii, J. Org. Chem., 1990, 55, 3917 CrossRef CAS.
- M. Nakahata, M. Imaida, H. Ozaki, T. Harada and A. Tai, Bull. Chem. Soc. Jpn., 1982, 55, 2186 CrossRef CAS.
- M. Hamamouchi and R. E. Prud'homme, J. Polym. Sci., Polym. Chem. Ed., 1988, 26, 1593 CrossRef.
- M. Sprecher, J. Biol. Chem., 1966, 241, 868 CAS.
| This journal is © The Royal Society of Chemistry 2000 |