Diterpenoids
James R.
Hanson
School of Chemistry, Physics and Environmental Science, University of Sussex, Brighton, Sussex, UK BN1 9QJ
First published on 19th December 2000
Abstract
Covering: 1999. Previous review: Nat. Prod. Rep., 2000, 17, 165.
1 Introduction
This report follows the pattern of its predecessors.1 The isolation and chemistry of the taxoids has again dominated the area. A number of studies have been reported on the chemotaxonomy of Taxus species. There have also been further studies on the structure–activity relationships of clerodanes as insect antifeedants. The diversity of the diterpenoid structures obtained from marine organisms and their biological activity has continued to attract interest.2 Acyclic and related diterpenoids
Geranylgeraniol and some oxygenated derivatives have been obtained![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 2 from the brown alga Bifurcaria bifurcata. Miriamin 1 has been identified
2 from the brown alga Bifurcaria bifurcata. Miriamin 1 has been identified![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 3 as a defensive diterpenoid produced by the eggs of a land slug of the Arion species. It has been shown to provide antifeedant protective action against predation by various species of beetle. The meroterpenoid chrysochlamic acid 2 from Chrysochlamys ulei has been reported
3 as a defensive diterpenoid produced by the eggs of a land slug of the Arion species. It has been shown to provide antifeedant protective action against predation by various species of beetle. The meroterpenoid chrysochlamic acid 2 from Chrysochlamys ulei has been reported![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 4 to inhibit DNA polymerase β. The antifungal agent, methoxybifuracerenone 3, which was previously reported as a constituent of Cystoseira amentacea, has been isolated
4 to inhibit DNA polymerase β. The antifungal agent, methoxybifuracerenone 3, which was previously reported as a constituent of Cystoseira amentacea, has been isolated![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 5 from C. tamariscifolia.
5 from C. tamariscifolia.
3 Bicyclic diterpenoids
3.1 Labdanes
The dinorlabdane 4 has been found![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 6 in Copaiba oil. The triene, juniperexcelsic acid 5, has been shown
6 in Copaiba oil. The triene, juniperexcelsic acid 5, has been shown![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 7 to be a cytotoxic constituent of the berries of Juniperus excelsa. The furanolabdadienoic acid 6 was amongst the diterpenoid constituents of Baccharis pingraea.8 Further investigations of Vitex rotundifolia have afforded
7 to be a cytotoxic constituent of the berries of Juniperus excelsa. The furanolabdadienoic acid 6 was amongst the diterpenoid constituents of Baccharis pingraea.8 Further investigations of Vitex rotundifolia have afforded![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 9 the spiro-ether 7.
9 the spiro-ether 7.
Labdanes have continued to be obtained from Croton species including Croton oblongifolius.10 Crotonadiol 8 was isolated![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 11 from the stems of C. zambesicus. The highly oxygenated 6,7-secolabdane, 2β-hydroxysaudinolide 9, was obtained
11 from the stems of C. zambesicus. The highly oxygenated 6,7-secolabdane, 2β-hydroxysaudinolide 9, was obtained![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 12 from Clutia richardiana. A number of bis-diterpenoids have been reported including moldenin 10 from Moldenhawera nutans.13 Further studies on the excoecarins from the wood of Excoecaria agalocha have been reported.14
12 from Clutia richardiana. A number of bis-diterpenoids have been reported including moldenin 10 from Moldenhawera nutans.13 Further studies on the excoecarins from the wood of Excoecaria agalocha have been reported.14
Studies on the partial synthesis of labdanes have included the synthesis of limonidilactone 11 from zamuranic acid.15
3.2 Clerodanes
The full 1H and 13C NMR assignments for stephalic acid 12 have been reported.16 Conyzalactone 13 has been obtained![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 17 from Conyza blinii. Examination of the Mexican plant Onoseris alata has afforded
17 from Conyza blinii. Examination of the Mexican plant Onoseris alata has afforded![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 18 some derivatives of 14. Further investigations of Teucrium polium, which has been used in Turkish folk medicine for the treatment of stomach complaints, has yielded
18 some derivatives of 14. Further investigations of Teucrium polium, which has been used in Turkish folk medicine for the treatment of stomach complaints, has yielded![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 19 teululin A 15. Clerodendrin I 16, which is a feeding stimulant for the turnip sawfly, has been isolated
19 teululin A 15. Clerodendrin I 16, which is a feeding stimulant for the turnip sawfly, has been isolated![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 20 from Clerodendron trichotomum. The insect antifeedant activity of scutecyprol B, obtained from Scutelaria rubicunda and of some other compounds derived by modification of fruticolone and scutelagin B, has been examined.21,22
20 from Clerodendron trichotomum. The insect antifeedant activity of scutecyprol B, obtained from Scutelaria rubicunda and of some other compounds derived by modification of fruticolone and scutelagin B, has been examined.21,22
A rearranged (4→2)-abeo-clerodane 17 has been obtained![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 23 from Aristolochia chamissonis. The cis languidulane carbon skeleton of salvimexicanolide 18, isolated from Salvia mexicana, may be derived from a clerodane.24 Jamesoniellide H 19 is a seco-clerodane which was obtained
23 from Aristolochia chamissonis. The cis languidulane carbon skeleton of salvimexicanolide 18, isolated from Salvia mexicana, may be derived from a clerodane.24 Jamesoniellide H 19 is a seco-clerodane which was obtained![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 25 from axenic cultures of the liverwort Jamesoniella autumnalis. Other cis clerodanes were isolated from the liverwort, Scapania nemorea.
25 from axenic cultures of the liverwort Jamesoniella autumnalis. Other cis clerodanes were isolated from the liverwort, Scapania nemorea.
The photochemical transformation of clerodanes including fruticolone has been examined.26 The structures of the products has led to the suggestion that some clerodanes may be photochemical artefacts. The photoxidation of the furan ring of hardwickiic acid to form a butenolide has been used in the clarification of the structure of some clerodanes.
4 Tricyclic diterpenoids
11α-Hydroxyabietadiene has been obtained![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 27 from Cryptomeria japonica (sugi). The structure 20 has been assigned
27 from Cryptomeria japonica (sugi). The structure 20 has been assigned![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 28 to gaultheric acid which was isolated from the roots of Gaultheria yunnanensis. A study
28 to gaultheric acid which was isolated from the roots of Gaultheria yunnanensis. A study![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 29 of the immunosuppressive constituents of Tripterygium wilfordii led to the isolation of triptobenzene L 21 whilst the cyclic peroxide 22 was obtained
29 of the immunosuppressive constituents of Tripterygium wilfordii led to the isolation of triptobenzene L 21 whilst the cyclic peroxide 22 was obtained![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 30 from the heartwood.
30 from the heartwood.
Mandarone A 23 was amongst the abietane diterpenoids isolated![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 31 from Clerodendron mandarinorum whilst the royleanone, bungone A 24, was detected
31 from Clerodendron mandarinorum whilst the royleanone, bungone A 24, was detected![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 32 in the stems of C. bungei. Another royleanone 25 was obtained
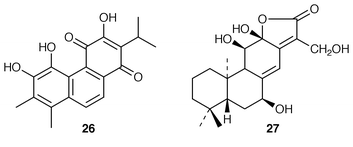
32 in the stems of C. bungei. Another royleanone 25 was obtained![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 33 from the roots of Salvia nutans whilst further investigations yielded similar compounds from the roots of S. glutinosa, S. austriaca, S. tomentosa and S. verticillata. Kronenquinone 26 is a constituent of S. kronenburgii.34 Trials have been reported
33 from the roots of Salvia nutans whilst further investigations yielded similar compounds from the roots of S. glutinosa, S. austriaca, S. tomentosa and S. verticillata. Kronenquinone 26 is a constituent of S. kronenburgii.34 Trials have been reported![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 35 on salvicine, a modified diterpenoid quinone, as a tumour inhibitor. Examination of the Chinese medicinal plant, Euphorbia fischeriana (Lang Du), has led
35 on salvicine, a modified diterpenoid quinone, as a tumour inhibitor. Examination of the Chinese medicinal plant, Euphorbia fischeriana (Lang Du), has led![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 36 to the isolation of langduin B 27, the structure of which was established by X-ray crystallography.
36 to the isolation of langduin B 27, the structure of which was established by X-ray crystallography.
A number of rearranged abietanes have been reported including standishinal 28 from Thuja standishii,37 dichroanal A 29 from Salvia dichroantha![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 38 and the dimeric diterpene, grandione 30 from Torreya grandis.39 The oidiolactones including 31 have been isolated
38 and the dimeric diterpene, grandione 30 from Torreya grandis.39 The oidiolactones including 31 have been isolated![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 40 from the fungus, Oidiodendron truncata. Some of these tetranorditerpene lactones have shown activity against pathogenic yeasts.41
40 from the fungus, Oidiodendron truncata. Some of these tetranorditerpene lactones have shown activity against pathogenic yeasts.41
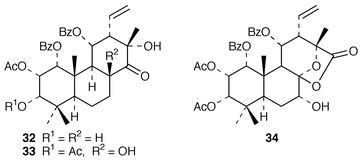
Some rearranged pimaranes have been obtained from Orthosiphon species including neoorthosiphol A 32 from O. aristatus,42 and staminol A 33 and the staminolactones (e.g. A, 34) from O. stamineus.43,44
The bromination of methyl dehydroabietate in the presence of montmorillonite K10![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 45 and the ozonolysis of methyl abietate
45 and the ozonolysis of methyl abietate![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 46 have been studied. Some modifications of rings B and C of podocarpic acid, directed at the synthesis of quassinoids, have been reported.47
46 have been studied. Some modifications of rings B and C of podocarpic acid, directed at the synthesis of quassinoids, have been reported.47
5 Tetracyclic diterpenoids
The crystal structure of linearol, ent-18-acetoxy-3β,7α-dihydroxykaurene, has been reported.48 The biotransformation of isosteviol and kaurenoic acid by Rhizopus species has been shown![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 49 to give the 7β- and 12-hydroxy derivatives. Steviol and some relatives have been isolated
49 to give the 7β- and 12-hydroxy derivatives. Steviol and some relatives have been isolated![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 50 from the rootbark of the marine mangrove tree, Bruguiera gymnorrhiza. The production of ent-kaurane-3,15-dione by cell cultures of the liverwort Jungermannia subulata and of carboxyatractyloside by cell suspension cultures of Atractylis gummifera have been reported.51,52
50 from the rootbark of the marine mangrove tree, Bruguiera gymnorrhiza. The production of ent-kaurane-3,15-dione by cell cultures of the liverwort Jungermannia subulata and of carboxyatractyloside by cell suspension cultures of Atractylis gummifera have been reported.51,52
Further investigations of Isodon (Rabdosia) species have led to the isolation of more hydroxylated kaurenoids including glabcensin V 35 from I. angustifoliusvarn. glabrescens,53 eriocalyxin C 36 from I. eriocalyx,54 rabdosianone I 37 from I. japonicus,55 lungshengenin G 38 from I. lungshengensis,56 and melissoidesin E 39 from I. melissoides.57 Some cytotoxic 11-oxygenated 8,9-secokauranes have been isolated![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 58 from a New Zealand liverwort, Lepidolaeria taylorii.
58 from a New Zealand liverwort, Lepidolaeria taylorii.
The total synthesis of the tetracyclic aphidicolane stemodane diterpenoids has been reviewed.59 A number of intermediates in the biosynthesis of aphidicolin have been isolated![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 60 from fermentations of Phoma betae treated with cytochrome P450 inhibitors. Studies on the biosynthesis of the gibberellins in maize
60 from fermentations of Phoma betae treated with cytochrome P450 inhibitors. Studies on the biosynthesis of the gibberellins in maize![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 61 and on the metabolism of kaurenoids and gibberellins in some mutants of the fungus Gibberella fujikuroi
61 and on the metabolism of kaurenoids and gibberellins in some mutants of the fungus Gibberella fujikuroi![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 62 have been reported.
62 have been reported.
6 Macrocyclic diterpenoids and their cyclization products
Further investigation of Croton oblongifolius has led![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 63 to the isolation of neocrotocembranal 40. The number of cembranes that have been obtained from marine organisms continues to rise. Cembranes that have been described include sartol acetate B 41 from a Sarcophyton species,64 brassicolide 42 from the coral, Nephthea brassica,65 sethukarailin 43 from Sinularia maxima,66 and further cembranolides from Pseudopterogorgia bipinnata.67
63 to the isolation of neocrotocembranal 40. The number of cembranes that have been obtained from marine organisms continues to rise. Cembranes that have been described include sartol acetate B 41 from a Sarcophyton species,64 brassicolide 42 from the coral, Nephthea brassica,65 sethukarailin 43 from Sinularia maxima,66 and further cembranolides from Pseudopterogorgia bipinnata.67
Investigations of the latex of the Euphorbiaceae have continued to yield diterpenes with the lathyrane, jatrophane and phorbol skeleta. These include euforboetol 44 which was isolated as its 3,5,17-triacetate from the irritant latex of a Portugese collection of Euphorbia boetica,68 some further jatrophanes from E. peplus,69 ingol esters from E. lactea,70 lathyranes from E. lathyris,71 and tumour promoting ingenol esters (milliamines) from E. leuconeura.72 The latter is a succulent plant which is sometimes used as an indoor decorative plant. Maprouneacin 45 is a daphnane with antihyperglycemic activity which was isolated![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 73 from Maprounea africana.
73 from Maprounea africana.
6.1 Taxanes
A comprehensive review of the naturally occurring taxanes has appeared.74 Recent advances in the medicinal chemistry of the taxanes has also been reviewed.75 The constituents of various members of the yew family have been reviewed. The distinction between the various Taxus species is difficult. The chemotaxonomy of Taxus species has been examined![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 76 in the light of their taxane content. The results of the analysis allowed a distinction to be made between the North American and Eurasian species. The occurrence of paclitaxel in Podocarpus gracilior (African fern pine) has been reported.77 A study of the seasonal variation of neutral and basic taxoids in the European yew, Taxus baccata, has revealed
76 in the light of their taxane content. The results of the analysis allowed a distinction to be made between the North American and Eurasian species. The occurrence of paclitaxel in Podocarpus gracilior (African fern pine) has been reported.77 A study of the seasonal variation of neutral and basic taxoids in the European yew, Taxus baccata, has revealed![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 78 a dependency on the location of the plant rather than the season.
78 a dependency on the location of the plant rather than the season.
A number of novel taxoids have been isolated. These include 46 from T. canadensis needles,79 1-hydroxytaxuspine C 47,80 taxezopidine J 48![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 81 and the rearranged taxane 49
81 and the rearranged taxane 49![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 82 from T. cuspidata. There have been a number of investigations into the taxanes of the Chinese yew, T. mairei. These have led to the isolation of the bicyclic compounds 50
82 from T. cuspidata. There have been a number of investigations into the taxanes of the Chinese yew, T. mairei. These have led to the isolation of the bicyclic compounds 50![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 83 and 51
83 and 51![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 84 and taxamain A 52.85 Further reports
84 and taxamain A 52.85 Further reports![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 86–90 on this species have included
86–90 on this species have included![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 91 the isolation of a 2(3→20)abeo-taxane 53. Other paclitaxel and brevifoliol analogues (e.g.54) have been obtained from T. media,92 and T. wallichiana.93
91 the isolation of a 2(3→20)abeo-taxane 53. Other paclitaxel and brevifoliol analogues (e.g.54) have been obtained from T. media,92 and T. wallichiana.93
A conformational study of the phenylisoserine side chain of paclitaxel has been reported.94 Further methods for the modification of the side chain have been described![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 95,96 together with a number of side chain variants.97 The synthesis and structure–activity relationships of taxoids possessing different substituents on the C-2 benzoyl group have been reported.98
95,96 together with a number of side chain variants.97 The synthesis and structure–activity relationships of taxoids possessing different substituents on the C-2 benzoyl group have been reported.98
The use of enzymatic methods in the structure modification of taxoids has been described.99 Some water soluble taxoids have been prepared.100 The modulation of multi-drug resistance in tumour cells by taxinine derivatives has been examined.101 The synthesis of 7-deoxy-6-hydroxypaclitaxel![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 102 and 10-deacetoxypaclitaxel
102 and 10-deacetoxypaclitaxel![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 103 have been reported. The structures of some rearrangement products of taxanes under various mineral and Lewis acid catalysed conditions have been elucidated.104–106 Other modifications of paclitaxel
103 have been reported. The structures of some rearrangement products of taxanes under various mineral and Lewis acid catalysed conditions have been elucidated.104–106 Other modifications of paclitaxel![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 107 and the synthesis of photoaffinity analogues
107 and the synthesis of photoaffinity analogues![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 108 have been described.
108 have been described.
7 Miscellaneous diterpenoids
The absolute configuration of the marine diterpenoid kalihinol A has been established by applying the exciton chirality methods to the p-bromobenzamide derivative 55.109 A number of spongiane and related diterpenoids have been described. A modified spongiane skeleton has been assigned![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 110 to the antibacterial diterpenoid, noscomin 56, which was isolated from Nostoc commune. The absolute stereochemistry has been determined
110 to the antibacterial diterpenoid, noscomin 56, which was isolated from Nostoc commune. The absolute stereochemistry has been determined![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 111 of anisodorin 5 57. This compound was obtained from the nudibranch, Anisodoris fontaini. Examination
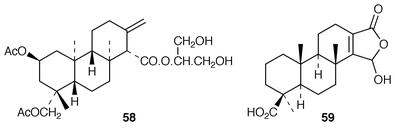
111 of anisodorin 5 57. This compound was obtained from the nudibranch, Anisodoris fontaini. Examination![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 112 of the Antarctic nudibranch, Austrodoris kerguelenensis, led to the isolation of the austrodorins A 58 and B. Spongiabutenolide A 59 was obtained
112 of the Antarctic nudibranch, Austrodoris kerguelenensis, led to the isolation of the austrodorins A 58 and B. Spongiabutenolide A 59 was obtained![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 113 from a Phillippines marine sponge.
113 from a Phillippines marine sponge.
An antibacterial acid 60 with a mulinane skeleton has been isolated![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 114 from Azorella compacta. Examination of the brown alga Stoechospermum marginatum has led
114 from Azorella compacta. Examination of the brown alga Stoechospermum marginatum has led![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 115 to the isolation of a further spatane 61 whilst a number of secospatanes such as secospatacetal A 62
115 to the isolation of a further spatane 61 whilst a number of secospatanes such as secospatacetal A 62![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 116 and dilkamural 63
116 and dilkamural 63![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 117 have been found in the brown alga, Dilophus okamurai.
117 have been found in the brown alga, Dilophus okamurai.
The total synthesis and chemical biology of the cytotoxic sarcodictyins have been reviewed.118 Massileunicellin A 64, which was obtained![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 119 from the gorgonian coral, Eunicella cavolinii, has an unusual structure with two ether bridges. Labiatin D 65 was obtained
119 from the gorgonian coral, Eunicella cavolinii, has an unusual structure with two ether bridges. Labiatin D 65 was obtained![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 120 from E. labiata. Examination of the gorgonian coral Briareum excavatum has led to the identification of a range of briarane diterpenoids known as the excavatolides
120 from E. labiata. Examination of the gorgonian coral Briareum excavatum has led to the identification of a range of briarane diterpenoids known as the excavatolides![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 121 or briaexcavatolides. A number of these show cytotoxic activity. These include briaexcavatolide A 66,122 briaexcavatolide G 67,123 and briaexcavatolide U 68.124 The violides, e.g.69, were isolated
121 or briaexcavatolides. A number of these show cytotoxic activity. These include briaexcavatolide A 66,122 briaexcavatolide G 67,123 and briaexcavatolide U 68.124 The violides, e.g.69, were isolated![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 125 from another Briareum species whilst further briarane diterpenoids have been obtained
125 from another Briareum species whilst further briarane diterpenoids have been obtained![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 126 from the gorgonian octacoral, Erythropodium caribaeurum. The malayenolides A–D, e.g. A, 70, were isolated
126 from the gorgonian octacoral, Erythropodium caribaeurum. The malayenolides A–D, e.g. A, 70, were isolated![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 127 from an Indonesian sea pen, Veretillum malayense. Xeniaol 71 is a xenicane diterpenoid which was obtained
127 from an Indonesian sea pen, Veretillum malayense. Xeniaol 71 is a xenicane diterpenoid which was obtained![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 128 from an Okinawan Xenia species of coral. Investigation of the West Indian sea whip, Pseudopterogorgia elisabethae, has afforded a number of diterpenoids including the sandresolides A 72 and B
128 from an Okinawan Xenia species of coral. Investigation of the West Indian sea whip, Pseudopterogorgia elisabethae, has afforded a number of diterpenoids including the sandresolides A 72 and B![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 129 and the amphilectanes, elisabatins A 73 and B.130 The oxazole pseudopteroxazole 1, 74, from the same species, was found
129 and the amphilectanes, elisabatins A 73 and B.130 The oxazole pseudopteroxazole 1, 74, from the same species, was found![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 131 to be a potent inhibitor of Mycobaterium tuberculosis. The isocyanide 75 was obtained
131 to be a potent inhibitor of Mycobaterium tuberculosis. The isocyanide 75 was obtained![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 132 from a Cribochalina species of sponge.
132 from a Cribochalina species of sponge.
Extraction of the rhizomes of Eurphorbia fischeriana afforded![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 133 the norditerpene, fischeria A 76. The aldovibsanines A 77 and B were obtained
133 the norditerpene, fischeria A 76. The aldovibsanines A 77 and B were obtained![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 134 from Viburnum odoratissimum whilst the unusual macrocyclic endoperoxide structure 78 was assigned
134 from Viburnum odoratissimum whilst the unusual macrocyclic endoperoxide structure 78 was assigned![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 135 to neovibsanin C which was obtained from V. aurabuki. Hatcherenone 79 which was isolated
135 to neovibsanin C which was obtained from V. aurabuki. Hatcherenone 79 which was isolated![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 136 from the German liverwort, Barbilophozia hatcheri, has a novel diterpenoid structure.
136 from the German liverwort, Barbilophozia hatcheri, has a novel diterpenoid structure.
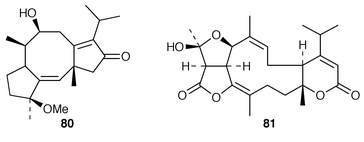
The fungus Alternariabrassicicola, which is responsible for a black spot disease of canola, has been shown![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 137 to produce the phytotoxin brassicicene A 80. The atranones, e.g.81, have been identified
137 to produce the phytotoxin brassicicene A 80. The atranones, e.g.81, have been identified![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 138 as metabolites of the toxigenic mould, Stachybotrys atra. Some further ryanoids have been isolated
138 as metabolites of the toxigenic mould, Stachybotrys atra. Some further ryanoids have been isolated![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 139 from the insecticidal plant, Ryania speciosa.
139 from the insecticidal plant, Ryania speciosa.
References
- J. R. Hanson, Nat. Prod. Rep., 2000, 17, 165 RSC.
- G. Culioli, V. Mesguiche, L. Piovetti and R. Valls, Biochem. Syst. Ecol., 1999, 27, 665 CrossRef CAS.
- F. C. Schroeder, A. Gonzalez, T. Eisner and J. Meinwald, Proc. Natl. Acad. Sci., USA, 1999, 96, 13620 CrossRef CAS.
- J.-Z. Deng, D.-A. Sun, S. R. Starck, S. M. Hecht, R. L. Cerny and J. R. Engen, J. Chem. Soc., Perkin Trans. 1, 1999, 1147 RSC.
- A. Bennamara, A. Abourriche, M. Berrada, M. Charrouf, N. Chaib, M. Boudouma and F. X. Garneau, Phytochemistry, 1999, 52, 37 CrossRef CAS.
- H. Monti, N. Tiliacos and R. Faure, Phytochemistry, 1999, 51, 1013 CrossRef CAS.
- G. Topcu, R. Erenler, O. Cakmak, C. B. Johansson, C. Celik, H. B. Chai and J. M. Pezzuto, Phytochemistry, 1999, 50, 1195 CrossRef CAS.
- G. A. Waechter, G. Montenegro and B. N. Timmermann, J. Nat. Prod., 1999, 62, 307 CrossRef CAS.
- M. Ono, M. Yamamoto, C. Masuoka, Y. Ito, M. Yamashita and T. Nohara, J. Nat. Prod., 1999, 62, 1532 CrossRef CAS.
- S. Roengsumran, A. Petsom, D. Sommit and T. Vilaivan, Phytochemistry, 1999, 50, 449 CrossRef CAS.
- B. T. Ngadjui, G. G. Folefoc, F. Keumedjio, E. Dongo, B. L. Sondengam and J. D. Connolly, Phytochemistry, 1999, 51, 171 CrossRef CAS.
- I. Muhammad, J. S. Mossa, H. H. Mirza and F. S. El-Feraly, Phytochemistry, 1999, 50, 1225 CrossRef CAS.
- J. P. David, J. M. David, S. W. Yang and G. A. Cordell, Phytochemistry, 1999, 50, 443 CrossRef CAS.
- T. Konishi, T. Konoshima, Y. Fujiwara, S. Kiyesawa, K. Miyahara and M. Nishi, Chem. Pharm. Bull., 1999, 47, 456 Search PubMed.
- I. S. Marcos, R. F. Moro, M. S. Carballiares and J. G. Urones, Tetrahedron Lett., 1999, 40, 2615 CrossRef CAS.
- E. Burgueno-Tapia, L. R. Hernandez, P. Joseph-Natham and M. Salmon, Magn. Reson. Chem., 1999, 37, 430 CrossRef CAS.
- L. Xu, D. Guo, J. Liu, J. Zheng, K. Koike, Z. Jia and T. Nikaido, Heterocycles, 1999, 51, 605 Search PubMed.
- E. E. Sigstad, M. del R. Cuenca, C. A. N. Catalan, T. E. Gedris and W. Herz, Phytochemistry, 1999, 50, 835 CrossRef CAS.
- E. Bedir, D. Tasdemir, I. Calis, O. Zerbe and O. Sticher, Phytochemistry, 1999, 51, 921 CrossRef CAS.
- K. Kawai, R. Nishida and H. Fukami, Biosci. Biotechnol. Biochem., 1999, 63, 1795 Search PubMed.
- M. Bruno, N. Vassalo and M. S. J. Simmonds, Phytochemistry, 1999, 50, 973 CrossRef CAS; M. Bruno, R. Ciriminna, F. Piozzi, S. Rosselli and M. S. J. Simmonds, Phytochemistry, 1999, 52, 1055 CrossRef CAS.
- B. Rodriguez, B. Rodriguez, M. C. de la Torre, M. S. J. Simmonds and W. M. Blaney, J. Nat. Prod., 1999, 62, 594 CrossRef CAS.
- M. D. Bomm, J. Zukerman-Schpector and L. M. X. Lopes, Phytochemistry, 1999, 50, 455 CrossRef CAS.
- B. Esquivel, N. Ramirez-Davalos and G. Espinosa-Perez, Heterocycles, 1999, 51, 1647 Search PubMed.
- W. Geis, B. Buschauer and H. Becker, Phytochemistry, 1999, 51, 643 CrossRef CAS; H. Tazaki, H. Becker and K. Nabata, Phytochemistry, 1999, 51, 743 CrossRef CAS.
- G. Fontana, G. Savona, N. Vivona and B. Rodriguez, Eur. J. Org. Chem., 1999, 2011 CrossRef CAS; M. Costa, C. M. A. Tanaka, P. M. Imamura and A. J. Marsaidi, Phytochemistry, 1999, 50, 117 CrossRef CAS.
- T. Ashitani, T. Iwaoka and S. Nagahama, Nat. Prod. Lett., 1999, 13, 169 Search PubMed.
- Z. Zhang, D. Guo, C. Li, J. Zheng, K. Koike, Z. Jia and T. Nikaido, J. Nat. Prod., 1999, 62, 297 CrossRef CAS.
- H. Duan, Y. Takaishi, H. Momota, Y. Ohmoto, T. Taki, Y. Jia and D. Li, J. Nat. Prod., 1999, 62, 1522 CrossRef CAS.
- F. Guo, M. Xi and Y. Li, Tetrahedron Lett., 1999, 40, 947 CrossRef CAS.
- T. Fan, Z. Min, G. Song, M. Iinuma and T. Tanaka, Phytochemistry, 1999, 51, 1005 CrossRef CAS.
- T. Fan, Z. Min and M. Iinuma, Chem. Pharm. Bull., 1999, 47, 1797 Search PubMed.
- G. Nagy, G. Gunther, I. Mathe, G. Blunden, M. H. Yang and T. A. Crabb, Phytochemistry, 1999, 51, 809 CrossRef CAS; G. Nagy, G. Gunther, I. Mathe, G. Blunden, M. H. Yang and T. A. Crabb, Phytochemistry, 1999, 52, 1103 CrossRef.
- G. Topcu and A. Ulubelen, J. Nat. Prod., 1999, 62, 1605 CrossRef CAS.
- J. S. Zhang, J. Ding, Q. M. Tang, M. Li, M. Zhao, L. J. Lu, L. J. Chen and S. T. Yuan, Bioorg. Med. Chem. Lett., 1999, 9, 2731 CrossRef CAS.
- C. T. Che, T. X. Zhou, Q. G. Ma, G. W. Qin, I. D. Williams, H. M. Wu and Z. S. Shi, Phytochemistry, 1999, 52, 117 CrossRef CAS.
- H. Ohtsu, M. Iwamoto, H. Ohishi, S. Matsunaga and R. Tanaka, Tetrahedron Lett., 1999, 40, 6419 CrossRef CAS.
- K. Kawazoe, M. Yamamoto, Y. Takaishi, G. Honda, T. Fujita, E. Sezik and E. Yesilada, Phytochemistry, 1999, 50, 493 CrossRef CAS.
- B. Galli, F. Gasparrini, V. Lanzotti, D. Misiti, R. Riccio, C. Villani, H. Guan Fu, Z. W. Ma and W. F. Yin, Tetrahedron, 1999, 55, 11385 CrossRef CAS.
- M. John, K. Krohn, U. Floerke, H. J. Aust, S. Draeger and B. Schulz, J. Nat. Prod., 1999, 62, 1218 CrossRef CAS.
- T. Hosoe, K. Nozawa, T. C. Lumley, R. S. Currah, K. Fukushima, K. Takizawa, M. Miyaji and K. Kawai, Chem. Pharm. Bull., 1999, 47, 1591 Search PubMed.
- H. Shibuya, T. Bohgaki and K. Ohashi, Chem. Pharm. Bull., 1999, 47, 911 Search PubMed.
- P. Stampoulis, Y. Tezuka, A. H. Banskota, K. Q. Tran, I. Saiki and S. Kadota, Tetrahedron Lett., 1999, 40, 4239 CrossRef CAS.
- P. Stampoulis, Y. Tazuka, A. H. Banskota, K. Q. Tran, I. Saiki and S. Kaduta, Org. Lett., 1999, 1, 1367 CrossRef CAS.
- A. M. Esteves, N. Narender, B. Gigante, M. J. Marcelo-Curto and F. Alvarez, Synth. Commun., 1999, 29, 275.
- C. dos Santos, C. R. S. de Rosso and P. M. Imamura, Synth. Commun., 1999, 29, 1903 CAS.
- R. C. Cambie, W. A. Denny, M. P. Hay, L. H. Mitchell, P. S. Rutledge and P. D. Woodgate, Aust. J. Chem., 1999, 52, 7 CrossRef CAS.
- T. Hokelek, E. Kilic and A. Oktemer, Anal. Sci., 1999, 15, 1167 ( Chem. Abstr. , 2000 , 132 , 78717 ) Search PubMed.
- B. H. de Oliveira, M. C. dos Santos and P. C. Leal, Phytochemistry, 1999, 51, 737 CrossRef CAS; E. A. Silva, J. A. Takahashi, M. A. D. Boaventura and A. B. Oliveira, Phytochemistry, 1999, 52, 397 CrossRef CAS.
- C. Subrahmanyam, B. V. Rao, R. S. Ward, M. B. Hursthouse and D. E. Hibbs, Phytochemistry, 1999, 51, 83 CrossRef CAS.
- H. Tazaki, T. Iwasaki, I. Nakasuga, K. Kobayashi, H. Koshino, M. Tanaka and K. Nabeta, Phytochemistry, 1999, 52, 1427 CrossRef CAS.
- T. Kephalas, F. Ali Karidis, K. Pantelia and D. Papadakis, Phytochemistry, 1999, 51, 53 CrossRef CAS.
- Q. S. Zhao, Z. W. Lin, B. Jiang, J. Wang and H. D. Sun, Phytochemistry, 1999, 50, 123 CrossRef CAS.
- S. N. Chen, J. M. Yue, S. Y. Chen, Z. W. Lin, G. W. Qin, H. D. Sun and Y. Z. Chen, J. Nat. Prod., 1999, 62, 782 CrossRef CAS.
- Y. Yamada, N. Sako, E. Ando, M. Yamada, H. Kikuzaki and T. Yamamoto, Biosci. Biotechnol. Biochem., 1999, 63, 524 Search PubMed.
- B. Jiang, Z. Q. Lu, A. J. Hou, Q. S. Zhao and H. D. Sun, J. Nat. Prod., 1999, 62, 941 CrossRef CAS.
- Q. S. Zhao, B. Jiang, Z. W. Lin and H. D. Sun, J. Asian Nat. Prod. Res., 1999, 1, 277 ( Chem. Abstr. , 2000 , 132 , 119847 ) Search PubMed.
- N. B. Perry, E. J. Burgess, S. H. Baek, R. T. Weavers, W. Geis and A. B. Mauger, Phytochemistry, 1999, 50, 423 CrossRef CAS.
- M. Toyota and M. Ihara, Tetrahedron, 1999, 55, 5641 CrossRef CAS.
- H. Oikawa, S. Ohashi, A. Ichihara and S. Sakakura, Tetrahedron, 1999, 55, 7541 CrossRef CAS.
- G. Davis, M. Kobayashi, B. O. Phinney, T. Lange, S. J. Croker, P. Gaskin and J. MacMillan, Plant Physiol., 1999, 121, 1037 CrossRef CAS.
- A. F. Barrero, J. E. Oltra, E. Cabrera, F. Reyes and M. Alvarez, Phytochemistry, 1999, 50, 1133 CrossRef CAS.
- S. Roengsumran, P. Singtothong, K. Pudham, N. Ngamrothanavanich, A. Petsom and C. Chaichantipyuth, J. Nat. Prod., 1999, 62, 1163 CrossRef CAS.
- T. Iawagwa, R. Nakashima, K. Takayama, H. Okamura, M. Nakatani, M. Doe and K. Shibata, J. Nat. Prod., 1999, 62, 1046 CrossRef CAS.
- C. Y. Duh, S. K. Wang, Y. L. Weng, M. Y. Chiang and C. F. Dai, J. Nat. Prod., 1999, 62, 1518 CrossRef CAS.
- Y. Venkateswarlu, K. V. Sridevi and M. R. Rao, J. Nat. Prod., 1999, 62, 756 CrossRef.
- A. D. Rodriguez, J. G. Shi and S. D. Huang, J. Nat. Prod., 1999, 62, 1228 CrossRef CAS.
- M. J. Ferreira and J. R. Ascenso, Phytochemistry, 1999, 51, 439 CrossRef CAS.
- J. Hohmann, A. Vasas, G. Gunther, G. Dombi, G. Blazso, G. Falkay, I. Mathe and G. Jerkovich, Phytochemistry, 1999, 51, 673 CrossRef CAS.
- A. A. Ahmed, M. Couladis, A. A. Mahmoud, A. De Adams and T. J. Mabry, Fitoterapia, 1999, 70, 140 CrossRef CAS.
- J. Hohmann, F. Evanics, A. Vasa, G. Dombi, G. Jerkovich and I. Mathe, J. Nat. Prod., 1999, 62, 176 CrossRef CAS.
- G. Vogg, E. Mattes, J. Rothenburges, N. Hertkorn, S. Achatz and H. Sandermann, Phytochemistry, 1999, 51, 289 CrossRef CAS.
- J. R. Carny, J. M. Krenisky, R. T. Williamson, J. Luo, T. J. Carlson, V. Littsu and J. L. Moswa, J. Nat. Prod., 1999, 62, 345 CrossRef.
- E. Baloglu and D. G. I. Kingston, J. Nat. Prod., 1999, 62, 1448 CrossRef CAS.
- I. Ojima, S. D. Kuduk and S. Chakravarty, Adv. Med. Chem., 1999, 4, 69 Search PubMed.
- V. S. Parmar, A. Jha, K. S. Bisht, P. Taneja, S. K. Singh, A. Kumar, R. Jain and C. E. Olsen, Phytochemistry, 1999, 50, 1267 CrossRef CAS; E. L. van Rozendaal, S. J. L. Kurstjens, T. A. Van Beek and R. G. Van den Berg, Phytochemistry, 1999, 52, 427 CrossRef CAS.
- R. Stahlut, G. Park, R. Petersen, W. Ma and P. Hylands, Biochem. Syst. Ecol., 1999, 27, 613 CrossRef.
- I. Hook, C. Poupat, A. Ahond, D. Guenard, F. Gueritto, M. T. Adeline, X. P. Wang, D. Dempsey, S. Breuillet and P. Potier, Phytochemistry, 1999, 52, 1041 CrossRef CAS.
- L. O. Zamir, J. Zhang, J. Wu, F. Sauriol and O. Mamer, J. Nat. Prod., 1999, 62, 1268 CrossRef CAS.
- Q. W. Shi, T. Oritani, T. Sugiyama, R. Murakami and T. Yamada, Biosci. Biotechnol. Biochem., 1999, 63, 924 Search PubMed.
- H. Shigemori, C. A. Sakurai, H. Hosoyama, A. Kobayashi, S. Kajiyama and J. Kobayashi, Tetrahedron, 1999, 55, 2553 CrossRef CAS.
- R. Murakami, Q. W. Shi, T. Horiguchi and T. Oritani, Biosci. Biotechnol. Biochem., 1999, 63, 1660 Search PubMed.
- Q. W. Shi, T. Oritani and T. Sugiyama, Phytochemistry, 1999, 50, 633 CrossRef CAS.
- Q. W. Shi, T. Oritani, T. Sugiyama and T. Yamada, Biosci. Biotechnol. Biochem., 1999, 63, 756 Search PubMed.
- S. J. Yang, J. M. Fang and Y. S. Cheng, Phytochemistry, 1999, 50, 127 CrossRef CAS.
- Q. W. Shi, T. Oritani and T. Sugiyama, Nat. Prod. Lett., 1999, 13, 81 Search PubMed.
- Q. W. Shi, T. Oritani and T. Sugiyama, Nat. Prod. Lett., 1999, 13, 105 Search PubMed.
- Q. W. Shi, T. Oritani, T. Sugiyama and T. Yamada, Nat. Prod. Lett., 1999, 13, 171 Search PubMed.
- Q. W. Shi, T. Oritani, T. Sugiyama and Q. Cheng, Nat. Prod. Lett., 1999, 13, 305 Search PubMed.
- Y. J. Chen, C. Y. Chen and Y. C. Shen, J. Nat. Prod., 1999, 62, 149 CrossRef CAS.
- Q. W. Shi, T. Oritani and T. Sugiyama, Nat. Prod. Lett., 1999, 13, 113 Search PubMed.
- B. Gabetta, N. Fuzzati, P. Orsini, F. Peterlongo, G. Appendino and D. G. van der Velde, J. Nat. Prod., 1999, 62, 219 CrossRef CAS.
- S. K. Chattopadhyay, V. Tripathi, R. P. Sharma, B. S. Joshi and R. Roy, Phytochemistry, 1999, 50, 131 CrossRef CAS.
- M. Milanesio, P. Ugliengo, D. Viterbo and G. Appendino, J. Med. Chem., 1999, 42, 291 CrossRef CAS.
- T. Yamaguchi, N. Harada, K. Pzaki, M. Hayashi, H. Arakawa and T. Hashiyama, Tetrahedron, 1999, 55, 1005 CrossRef CAS.
- P. G. Jagtap and D. G. I. Kingston, Tetrahedron Lett., 1999, 40, 189 CrossRef CAS.
- E. J. Roh, C. E. Song, D. Kim, H. O. Pae, H. T. Chung, K. S. Lee, K. B. Chai, C. O. Lee and S. Un Choi, Bioorg. Med. Chem., 1999, 7, 2115 CrossRef CAS.
- I. Ojima, T. Wang, M. L. Miller, S. Lin, C. P. Borella, X. Geng, P. Pera and R. J. Bernacki, Bioorg. Med. Chem. Lett., 1999, 9, 3423 CrossRef CAS.
- D. Lee and M. J. Kim, Org. Lett., 1999, 1, 925 CrossRef CAS.
- T. Yamaguchi, N. Harada, K. Ozaki, H. Arakawa, K. Oda, N. Nakanishi, K. Tsujihara and T. Hashiyama, Bioorg. Med. Chem. Lett., 1999, 9, 1639 CrossRef CAS.
- H. Hosoyama, H. Shigemori, A. Tomida, T. Tsuruo and J. Kobayashi, Bioorg. Med. Chem. Lett., 1999, 9, 389 CrossRef CAS.
- M. D. Wittman, T. J. Alstadt, J. F. Kadow, D. M. Vyas, K. Johnson, C. Fairchild and B. Long, Tetrahedron Lett., 1999, 40, 4943 CrossRef CAS.
- D. Dutta, H. Hadd, D. G. van der Velde and G. I. Georg, Bioorg. Med. Chem. Lett., 1999, 9, 3277 CrossRef CAS.
- L. O. Zamir, A. D. Cherestes, A. Nikolakakis, F. Sauriol and O. Mamer, Tetrahedron Lett., 1999, 40, 7917 CrossRef CAS.
- Q. Cheng, T. Oritani and T. Horiguchi, Tetrahedron, 1999, 55, 12099 CrossRef CAS.
- H. Hirokazu, H. Shigemori and J. Kobayashi, Tetrahedron Lett., 1999, 40, 2149 CrossRef.
- A. A. L. Gunatilaka, F. D. Ramdayal, M. H. Sarragiotto, D. G. I. Kingston, D. L. Sackett and E. Hamel, J. Org. Chem., 1999, 64, 2694 CrossRef CAS.
- I. Ojima, P. Y. Bounard and D. G. Ahern, Bioorg. Med. Chem. Lett., 1999, 9, 1189 CrossRef CAS.
- M. Shimomura, H. Miyaoka and Y. Yamada, Tetrahedron Lett., 1999, 40, 8015 CrossRef CAS.
- B. Jaki, J. Orjala and O. Sticher, J. Nat. Prod., 1999, 62, 502 CrossRef CAS.
- N. Ungur, M. Gavagnin, E. Mollo and G. Cimino, Tetrahedron: Asymmetry, 1999, 10, 1635 CrossRef.
- M. Gavagnin, A. De Napoli, F. Castelluccio and G. Cimino, Tetrahedron Lett., 1999, 40, 8471 CrossRef CAS.
- S. S. Mitchell, K. M. Harper and D. J. Faulkner, Tetrahedron, 1999, 55, 10887 CrossRef CAS.
- G. A. Waechter, G. Matooq, J. J. Hoffmann, W. M. Maiese, M. P. Singh, G. Montenegro and B. M. Timmermann, J. Nat. Prod., 1999, 62, 1319 CrossRef CAS.
- S. De Rosa, C. Iodice, M. Khalaghdoust, S. Oryan and A. Rustaiyan, Phytochemistry, 1999, 51, 1009 CrossRef CAS.
- H. Yamase, K. Umemoto, T. Ooi and T. Kusumi, Chem. Pharm. Bull., 1999, 47, 813 Search PubMed.
- M. Ninomiya, H. Hirohara, J. Onishi and T. Kusumi, J. Org. Chem., 1999, 64, 5436 CrossRef CAS.
- K. C. Nicolaou, J. Pfefferkorn, J. Xu, N. Wissinger, T. Ohshima, S. Kim, S. Hosokawa, D. Vourloumis, F. van Delft and T. Li, Chem. Pharm. Bull., 1999, 47, 1199 Search PubMed.
- I. Mancini, G. Guella, H. Zibrowius, D. Laurent and F. Pietra, Helv. Chim. Acta, 1999, 82, 1681 CrossRef CAS.
- C. Kakonikos, C. Vagias and V. Roussi, Nat. Prod. Lett., 1999, 13, 89 Search PubMed.
- J. E. Neve, B. J. McCool and B. F. Bowden, Aust. J. Chem., 1999, 52, 359 CAS.
- J. H. Sheu, P. J. Sung, J. H. Su, H. Y. Liu, C. Y. Duh and M. Y. Chiang, Tetrahedron, 1999, 55, 14555 CrossRef CAS.
- P. J. Sung, J. H. Su, G. H. Wang, S. F. Lin, C. Y. Duh and J. H. Sheu, J. Nat. Prod., 1999, 62, 457 CrossRef CAS.
- J. H. Sheu, P. J. Sung, J. H. Su, G. H. Wang, C. Y. Duh, Y. C. Shen, M. Y. Chiang and I. T. Chen, J. Nat. Prod., 1999, 62, 1415 CrossRef CAS.
- T. Iwagawa, K. Takayama, H. Okamura, M. Nakatani, M. Doe, K. Takemura and M. Shiro, Heterocycles, 1999, 51, 2619 Search PubMed.
- D. Maharaj, K. O. Pascoe and W. F. Tinto, J. Nat. Prod., 1999, 62, 313 CrossRef CAS.
- X. Fu, F. J. Schmitz and G. C. Williams, J. Nat. Prod., 1999, 62, 103.
- H. Miyaoka, M. Nakano, K. Iguchi and Y. Yamada, Tetrahedron, 1999, 55, 12977 CrossRef CAS.
- A. D. Rodriguez, C. Ramirez and I. I. Rodriguez, Tetrahedron Lett., 1999, 40, 7627 CrossRef CAS.
- A. D. Rodriguez, C. Ramirez and I. I. Rodriguez, J. Nat. Prod., 1999, 62, 997 CrossRef CAS.
- A. D. Rodriguez, C. Ramirez and I. I. Rodriguez, Org. Lett., 1999, 1, 527 CrossRef CAS.
- M. L. Ciavatta, A. Fontana, R. Puliti, G. Scognamiglio and G. Cimino, Tetrahedron, 1999, 55, 12629 CrossRef CAS.
- Y. Pei, K. Koike, B. Han, J. Zia and T. Nikaido, Tetrahedron Lett., 1999, 40, 951 CrossRef CAS.
- M. Kubo, I. S. Chen, H. Minami and Y. Fukuyama, Chem. Pharm. Bull., 1999, 47, 295 Search PubMed.
- M. Kubo, H. Minami, E. Hayashi, M. Kodama, K. Kawazu and Y. Fukuyama, Tetrahedron Lett., 1999, 40, 6261 CrossRef CAS.
- F. Nagashima, Y. Murakami and Y. Asakawa, Chem. Pharm. Bull., 1999, 47, 138 Search PubMed.
- S. L. Mackinnon, P. Keifer and W. A. Ayer, Phytochemistry, 1999, 51, 215 CrossRef CAS.
- S. F. Hinkley, J. Jiang, E. P. Mazzola and B. B. Jarvis, Tetrahedron Lett., 1999, 40, 2725 CrossRef CAS.
- L. Ruest, C. Berthelette, M. Dodier, L. Dube and D. St. Martin, Can. J. Chem., 1999, 77, 12 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2001 |