Simple indole alkaloids and those with a nonrearranged monoterpenoid unit
Satoshi Hibino and Tominari Choshi
Faculty of Pharmacy and Pharmaceutical Sciences, Fukuyama University, Fukuyama, Hiroshima 729-0292, Japan
First published on 9th January 2001
Abstract
Covering: January 1999 to December 1999. Previous review: 2000, 17, 175.
 Satoshi Hibino Satoshi Hibino | Satoshi Hibino was born in 1945 in Aichi, Japan. He graduated in 1972 from the Graduate School of Pharmaceutical Sciences, Tohoku University and he received his PhD degree from the same university under the supervision of Professor Tetsuji Kametani. After two years of postdoctoral work (1975–1977) with Professor Steven M. Weinreb (Dept. Chem., Penn State U.) at Fordham University, he worked at Tokyo Pharmaceutical University as a faculty member (1977–1982). In 1982 he moved to the Faculty of Pharmacy and Pharmaceutical Sciences, Fukuyama University and then in 1986 became a full professor. His main research interests are the synthesis of biologically active natural products, especially nitrogen containing heterocyclic compounds based on electrocyclic reactions |
 Tominari Choshi Tominari Choshi | Tominari Choshi was born in 1964 in Hiroshima, Japan. He graduated in 1989 from the Graduate School of Pharmaceutical Sciences, Okayama University and then he received his MS Degree. He obtained his PhD degree in 1997 from Tohoku University under the supervision of Professor Keiichiro Fukumoto. He became a faculty member of Faculty of Pharmacy and Pharmaceutical Sciences, Fukuyama University in 1992. Currently he is a full-time lecturer in organic chemistry. His main research interests are the synthesis of biologically active natural products, especially nitrogen containing heterocyclic compounds based on electrocyclic reactions. |
1 Introduction
This review covers the literature on simple indole alkaloids and those with a nonrearranged monoterpenoid unit from the beginning of 1999 up to the end of 1999. The subject is developed along the lines of our respected predecessors (Saxton, Fukumoto, Ihara, Toyota, Lounasmaa, and Tolvanen). Subheadings along with the title have been established by Lounasmaa and Tolvanen. We applied these subheadings in the review, but a location of a new and/or synthetic alkaloid might be slightly different from that of the former review. Marine natural products have been reviewed in these Reports by Faulkner![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 1 and peptide alkaloids have also been surveyed in this series by Lewis.2 There will inevitably be some overlap with marine alkaloids and peptide alkaloids containing the indole moiety. New approaches to the total synthesis of manzamine A, ircinal A and related compounds based on a highly efficient Diels–Alder reaction have been reviewed by Nakagawa.3
1 and peptide alkaloids have also been surveyed in this series by Lewis.2 There will inevitably be some overlap with marine alkaloids and peptide alkaloids containing the indole moiety. New approaches to the total synthesis of manzamine A, ircinal A and related compounds based on a highly efficient Diels–Alder reaction have been reviewed by Nakagawa.32 Simple indole alkaloids
2.1 Non-tryptamines
The simple, new indole alkaloid, 3,6-dibromoindole 1 possessing antibacterial activity, has been isolated from the acsidian Distaplia regina.4 Two 1H-indole-3-acetonitrile glycosides, capparilosides A 2a and B 2b have been newly isolated from the methanolic extract of the mature fruits of Capparis spinosa.5 Their structures were established by spectral analysis and chemical transformation. 3-(3-Indolyl)acrylamide 3a has been found from the acetone extracts of the red algae Chondria atropurpurea together with chondriamide A 3b isolated previously.6 An antifungal indole, fistulosin (2-octadecyl-3-hydroxyindole) 4, which shows high activity against Fusarium oxysporum primarily inhibiting protein synthesis, has been found in the roots of Welsh onion (Allium fistulosum L.).7The structures of APHE 1–4 (pyrazolo[1,5-b]isoquinolin-9(1H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-one ring system), produced by actinomycete Streptverticillium griseocarneum, have been reformulated on the basis of the synthesis of pyrazolo[1,5-b]isoquinoline by Kelly
)-one ring system), produced by actinomycete Streptverticillium griseocarneum, have been reformulated on the basis of the synthesis of pyrazolo[1,5-b]isoquinoline by Kelly![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 8 as pimprinethine 5a, WS-30581 A 5b, pimprinine 5c and WS-30581 B 5d, respectively, which have been isolated from strains of microorganisms
8 as pimprinethine 5a, WS-30581 A 5b, pimprinine 5c and WS-30581 B 5d, respectively, which have been isolated from strains of microorganisms![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 9 very similar to the one that produces the APHEs.
9 very similar to the one that produces the APHEs.
Total syntheses of three simple indole alkaloids, 2,4-dimethylindole 6a, 4-(hydroxymethyl)-2-methylindole 6b, and 4-(methoxymethyl)-2-methylindole 6c, isolated from two species of European Basidomycetes (Tricholoma virgatum and Tricholomasciodes),10 have been accomplished by a palladium-phosphine-catalyzed N-heteroannulation as the key step (Scheme 1).11
 | ||
| Scheme 1 Reagents: i, Pd(OAc)2, PPh3, CO, 70 °C, MeCN; ii, DIBAL-H; iii, MeI, KOH. | ||
The total synthesis of polyamine toxin of HO-4166 12, isolated from the venomous spider Hololena curta,12 has been completed in twelve steps from 1,3-diaminopropane in 41% overall yield by using the 2-nitrobenzenesulfonamide (Ns) as both a protecting and activating group. The deprotection of the Ns group was performed by treatment of a new resin prepared from Merrified resin with p-hydroxytrityl alcohol (Scheme 2).13
 | ||
| Scheme 2 Reagents: i, PivCl, Et3N, CH2Cl2, rt; ii, CsCO3, MeCN, 60 °C; iii, SOCl2, MeOH, rt; iv, resin, i-Pr2NEt, CH2Cl2, rt; v, HSCH2CH2OH, DBU, DMF, rt; vi, TFA, CH2Cl2. | ||
Two new batzelline analogues, secobatzellines A 13a and B 13b, have been isolated from a deep-water marine sponge of the genus Batzella. They inhibit the phosphatase activity of calcineuron, and show in vitro cytotoxicity against P-388 and A-549 cell lines. Secobatzelline A also inhibits the peptidase activity of CPP32. These compounds are probable precursors of pyrroloiminoquinone alkaloids.14
Two new insecticidal agents, nodulisporic acids A114a and A214b have been isolated from a Nodulisporium sp.15 They exhibit similar biological profiles to closely related nodulisporic acid A.16
 | ||
| Scheme 3 Reagents: i, NaHMDS, −78 °C; ii, PNBSA, −78 °C to rt; iii, copper(I) triflate; iv, hydrolysis; v, MsCl, Et3N; vi, DBU, CH2Cl2. | ||
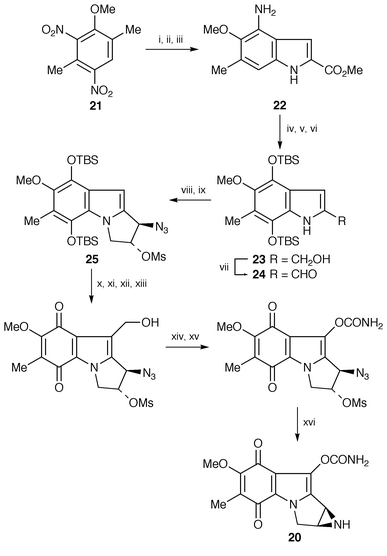 | ||
| Scheme 4 Reagents: i, (COOMe)2, t-BuOK, Et2O; ii, SnCl2, MeOH; iii, H2, Pd-C, EtOH; iv, Fremy’s salt, 0.2 M NaH2PO4, acetone; v, H2, Pd-C, then TBSCl, imidazole, CH2Cl2; vi, DIBAL-H, THF; vii, MnO2, CH2Cl2; viii, diisopropylvinylsulfonium triflate, NaH, THF, 0 °C, then NaN3, acetone, H2O; ix, MsCl, Et3N, CH2Cl2; x, POCl3, DMF; xi, PCC, CH2Cl2; xii, NaBH4, MeOH; xiii, O2; xiv, phenyl chloroformate, pyridine; xv, NH3, CH2Cl2; xvi, PPh3, Et3N, THF, H2O. | ||
The first synthesis of optically pure biscarbazole has been reported. Optically pure dimeric O-demethylmurrayafoline A 29a and 8,8′-demethylclausenamine-A 29b have been synthesized via resolution of the corresponding camphor sulfonates of the racemates. The absolute configuration of (+)-29a, (+)-29b and (−)-29a, (−)-29b as (aR) and (aS![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ), respectively, has been established by X-ray crystallographic and CD spectral analyses.25
), respectively, has been established by X-ray crystallographic and CD spectral analyses.25
Improved total synthesis of carbazomycins A 30a and B 30b, isolated from Streptomyces ehimense,26 have been completed. The key steps are a C–C bond formation by electrophilic aromatic substitution at the fully functionalized arylamine 32 with the tricarbonyliron-complexed cyclohexadienyl cation 31 and a subsequent C–N bond formation by oxidative cyclization of the intermediate tricarbonyliron–cyclohexadiene complexes 33 in air (Scheme 5).27 This type of transition metal promoted cyclization procedure for the construction of carbazole framework has been further applied to the synthesis of the marine alkaloid hyellazole 35, isolated from the blue–green alga Hyella caespitosa,28 by the same group.29
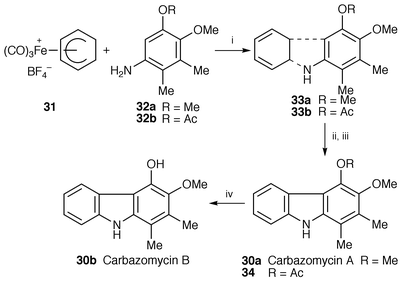 | ||
| Scheme 5 Reagents: i, MeCN, air, 25 °C; ii, Me3NO, Me2CO, 56 °C; iii, 10% Pd-C, o-xylene, 145 °C; iv, LiAlH4, THF, 25 °C. | ||
A concise synthesis of furostifoline 36, isolated from Murraya eucrestifolia,30 has been completed through a five step procedure based on a palladium(0)-catalyzed cross-coupling reaction (Scheme 6).31
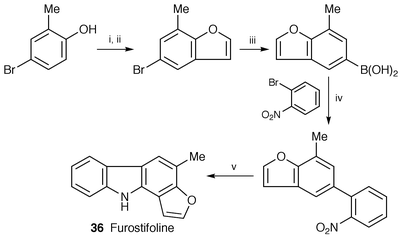 | ||
| Scheme 6 Reagents: i, BrCH2CH(OEt)2, K2CO3, DMF, 100 °C; ii, H3PO4, P2O5, PhCl, 140 °C; iii, THF, −78 °C, n-BuLi, B(OBu)3, H2O; iv, Pd(0), Na2CO3, DME, H2O, reflux; v, P(OEt)3, reflux. | ||
Total syntheses of cryptotackieine 38 (also named neocryptolepine, indolo[2,3-b]quinoline ring system) and cryptosanguinolentine 39 (also named isocryptolepine indolo[3,2-c]quinoline ring system), isolated from Cryptolepissanguinolenta,34 have been developed by using the key common compound 40. The intramolecular aza-Wittig reaction of 40 provided cryptotackieine 38. By contrast, cryptosanguinolentine 39 has been obtained by a nitrene insertion process within 40 followed by reduction (Scheme 7).35 Cryptotackieine 38 has also been synthesized by the same group through the aza-Wittig–electrocyclic ring closure strategy.36
 | ||
| Scheme 7 Reagents: i, microwave, nitrobenzene; ii, MeI, DMF, 60 °C; iii, H, Pd/C, rt; iv, NaNH2, H2SO4, H2O, NaN3; v, microwave, Me3P, nitrobenzene; vi, o-xylene; vii, Red-Al, reflux. | ||
The first total synthesis of cryptomisrine 41 (dimeric indolo[3,2-b]quinoline), isolated from Cryptolepis sanguinolenta,33 has been achieved regioselectively.37 The key step is based on a convergent methodology which involves a new halogen-dance reaction![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 38 (42→43 and 43→44) in the quinoline ring followed by Suzuki cross-coupling reaction (Scheme 8).
38 (42→43 and 43→44) in the quinoline ring followed by Suzuki cross-coupling reaction (Scheme 8).
 | ||
| Scheme 8 Reagents: i, LDA, THF, −78 °C, HCOOEt; ii, LDA, THF, −78 °C, 42; iii, Pd(PPh3)4, 2 M K2CO3, EtOH, toluene, Ar, reflux; iv, MnO2, toluene, reflux; v, pyridinium chloride, 160 °C, NH4OH. | ||
2.2 Non-isoprenoid tryptamines
A new N-fatty acyl tryptamine, cheritamine 45, along with thirty-two known compounds have been obtained from the stems of Annona cherimola.39 A further eight tryptamine derived amides, N-palmitoyltryptamine 46a, N-stearoyltryptamine 46b, N-arachidoyltryptamine 46c, N-behenoyltryptamine 46d, N-tricosanoyltryptamine 46e, N-lignoceroyltryptamine 46f, N-pentacosanoyltryptamine 46g, and N-cerotoyltryptamine 46h, have been isolated from the seeds of Rollinia mucosa.40 Their structures have been characterized and identified by physical and spectral data. Two new bioactive indole alkaloids, bufobutanoic acid 47a and bufopyramide 47b have been isolated from the Chinese traditional drug Ch’ an Su, prepared from the skin secretions of the local toads such as Bufo bufo garigarizans or Bufo melanostrictus. Both compounds exhibit cytotoxicity against murine P388 lymphocytic leukemia cells with IC50 values of 22 μg mL−1 and 26 μg mL−1, respectively.41 A sulfur-containing indole alkaloid, glypetelotine 48 has been isolated from the leaves of the endemic Vietnamese plant Glycosmis petelotti. The structure of 48 has been established to be S-methyl N,N-2[(1H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-indol-3-ethyl]methyl thiocarbamate by spectroscopic analysis.42
)-indol-3-ethyl]methyl thiocarbamate by spectroscopic analysis.42A new total synthesis of psilocin 49, isolated from the mushroom species Psilocybemexicana![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 43 as a minor hallucinogenic component, has been achieved by employing the directed regioselective iodination and the Heck-type coupling reaction (50→51) as key steps for the construction of the indole ring (Scheme 9).44
43 as a minor hallucinogenic component, has been achieved by employing the directed regioselective iodination and the Heck-type coupling reaction (50→51) as key steps for the construction of the indole ring (Scheme 9).44
 | ||
| Scheme 9 Reagents: i, t-BuLi, Et2O, −78 °C→25 °C, I2, THF, −78°→25 °C; ii, 2,5-dihydro-2,5-dimethoxyfuran, Pd(OAc)2, i-Pr2NEt, PhCH2NEt3Cl, DMF, 80 °C; iii, TFA, CH2Cl2, 0 °C→rt. | ||
Two diastereomers that are antipodal at C-7 of 3-oxo-7-hydroxy-3,7-secorhynchophylline 52 isolated from Uncaria attenuata Korth in Thailand, have been prepared, and semi-synthetic 52 has been separated by chiral column chromatography. The absolute configuration at C-7 was elucidated by comparison of the CD spectra with those of the known 3-hydroxy-2-oxotryptophan.45 Further the absolute stereochemistry at the C-3′ position of maremycin A 53a and B 53b, isolated from the culture broth of marine Streptomyces, was elucidated to be (S![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) and (R), respectively, based on the analysis of their CD spectra.45 Subsequently absolute configurations of four 3-hydroxyindole alkaloids, convolutamydines A 54a, B 54b, C, 54c and D 54d, isolated from marine bryozoan by Kamano group,46 were suggested to be (R)-form based on a negative Cotton effect in the CD spectra.45
) and (R), respectively, based on the analysis of their CD spectra.45 Subsequently absolute configurations of four 3-hydroxyindole alkaloids, convolutamydines A 54a, B 54b, C, 54c and D 54d, isolated from marine bryozoan by Kamano group,46 were suggested to be (R)-form based on a negative Cotton effect in the CD spectra.45
An indole alkaloid, racemic convolutamydine A 54a, isolated from marine bryozoan Amathia convoluta,46 has been synthesized starting from 4,6-dibromoisatin 55 (Scheme 10).47
 | ||
| Scheme 10 Reagents: i, acetone, 15% KOH, EtOH; ii, acetone, PhCH2N+Me3−OH. | ||
(+)-Alantrypinone is a structurally interesting natural product isolated from the fungus Pennicillium thymicola. Total synthesis of the enantiomer 56 of alantrypinone along with its epimer has been established in ten steps, starting from the reaction of isatoic anhydride 57 with (S![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-(−)-tryptophan methyl ester 58 (Scheme 11).48
)-(−)-tryptophan methyl ester 58 (Scheme 11).48
![Reagents: i, toluene, reflux; ii, H2O, CH2Cl2, NaHCO3; iii, PPh3, I2, i-Pr2NEt; iv, [Me3AlSPh]Li, THF; v, piperidine, THF; vi, m-CPBA, CH2Cl2; vii, benzene, PPh3, reflux; viii, TFA; ix, NBS, TFA; x, Pd-C, H2, MeOH.](/image/article/2001/NP/b004055j/b004055j-s11.gif) | ||
| Scheme 11 Reagents: i, toluene, reflux; ii, H2O, CH2Cl2, NaHCO3; iii, PPh3, I2, i-Pr2NEt; iv, [Me3AlSPh]Li, THF; v, piperidine, THF; vi, m-CPBA, CH2Cl2; vii, benzene, PPh3, reflux; viii, TFA; ix, NBS, TFA; x, Pd-C, H2, MeOH. | ||
An enantioselective total synthesis of the oxindole alkaloid (−)-horsfiline 59, isolated from Malaysian medicinal plant Horsfilidea superba,49 has been developed on the basis of asymmetric nitroolefination (60→62) using the chiral nitro-enamine 61 as the key step (Scheme 12).50
 | ||
| Scheme 12 Reagents: i, n-BuLi, toluene, Et2O, 61; ii, 1 M HCl, MeOH; iii, NaBH4, dioxane, MeOH; iv, NaH, DMF, PHCH2Cl; v, NaNO2, AcOH, DMSO; vi, Et3N, DPPA, EtOH; vii, O3, EtOH, −78 °C, Me2S, NaBH4, MeOH; viii, Et3N, MsCl, CH2Cl2, NaH, THF; ix, KOH, NH2NH2, HOCH2CH2OH, HCOOH, HCHO; x, Li, NH3. | ||
The formal total synthesis of (±)-pedunclarine 63, the principal alkaloid of the Tasmania shurab Aristelia pedunclaris, has been accomplished by the construction of the 6-azabicyclo[3.2.1]octane ring system based on the Cr(0)-promoted higher order cycloaddition as the key step.51
A cyclized didemnimide alkaloid 64 from the Caribbean Ascidian Didemnumconchyliatum has been isolated.52 The structure of this alkaloid was identical with isogranulatimide 64, recently isolated from the ascidian Didemnum granulatum by the Andersen group.53
Total syntheses of amauromine 69 (isolated from Amauroascus sp. 6237![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 58) and 5-N-acetylardeemin 70 (isolated from the fungus Aspergillus fischerii
58) and 5-N-acetylardeemin 70 (isolated from the fungus Aspergillus fischerii![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 59) have been completed on the basis of the kinetic stereoselective conversion of 71→72a, followed by the reverse prenylation (conversion of 72a→73) for the construction of the common hexahydropyrrolo[2,3-b]indole 73 (Scheme 13).60 Total synthesis of spirotryprostatin A 75, isolated from the fermentation broth of Aspergillus fumigatus,61 has been accomplished in nine steps. The key step is the oxidative rearrangement of the β-carboline ring 76 to the oxindole 77 by NBS (Scheme 14). The structure activity relations of 75 and its related compounds for cell cycle inhibition has also been described.62
59) have been completed on the basis of the kinetic stereoselective conversion of 71→72a, followed by the reverse prenylation (conversion of 72a→73) for the construction of the common hexahydropyrrolo[2,3-b]indole 73 (Scheme 13).60 Total synthesis of spirotryprostatin A 75, isolated from the fermentation broth of Aspergillus fumigatus,61 has been accomplished in nine steps. The key step is the oxidative rearrangement of the β-carboline ring 76 to the oxindole 77 by NBS (Scheme 14). The structure activity relations of 75 and its related compounds for cell cycle inhibition has also been described.62
 | ||
| Scheme 13 Reagents: i, N-phenylselenophthalimide, pyridinium toluene-p-sulfonate; ii, MeOTf, 2,6-di(tert-butyl)pridine, prenyltri(n-butyl)stannane, CH2Cl2, −78 °C→reflux; iii, separation (cryst); iv, NaOH, THF, MeOH, H2O, reflux; v, TMSI, MeCN, 0 °C; vi, 74, BOP-Cl, Et3N, CH2Cl2. | ||
 | ||
| Scheme 14 Reagents: i, TFA, 4 Å ms, CH2Cl2, 0 °C→rt; ii, NBS, AcOH, H2O, THF; iii, TFA, CH2Cl2; iv, Boc2O, MeCN, Et3N, reflux; v, N-Troc-Proline acid chloride, Et3N, CH2Cl2, 0 °C→rt; vi, Zn, THF, MeOH, NH4Cl; vii, NaIO4, H2O, MeOH; viii, toluene, reflux; ix, RhCl3·3H2O, EtOH, reflux. | ||
A total synthesis of the acyl-CoA: cholesterol acyltransferase inhibitor gypsetin 78, isolated from N. gypsea var. incurvata IFO9228,63 has been completed on the basis of the introduction of a reversed prenyl group at the 2-position of N-phthaloyltryptophan methyl ester (79→80), followed by the dimethyldioxirane-promoted double-oxidative cyclization of the diketopiperazine (82→78) (Scheme 15).64 Total syntheses of deoxybrevianamide E 83, a metabolite of Aspergillus ustus![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 65 and brevianamide E 84, isolated from the culture of Penicilliumbrevicompactum
65 and brevianamide E 84, isolated from the culture of Penicilliumbrevicompactum![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 66 have also been achieved from 81.64 Furthermore, total synthesis of tryprostatin B 85, isolated from Aspergillus fumigatus,61a,67 has also been accomplished by the same group, starting from 79 (Scheme 16).64
66 have also been achieved from 81.64 Furthermore, total synthesis of tryprostatin B 85, isolated from Aspergillus fumigatus,61a,67 has also been accomplished by the same group, starting from 79 (Scheme 16).64
 | ||
| Scheme 15 Reagents: i, t-BuOCl, Et3N, −78 °C; ii, prenyl 9-BBN, −78 °C; iii, NH2NH2, EtOH, rt; iv, 140 °C; v, dimethyldioxirane, CH2Cl2, acetone, −78 °C→0 °C; vi, Boc-proline, BOP-Cl, CH2Cl2, −78 °C→0 °C; vii, TFA, CH2Cl2, rt, then NH3, MeOH, reflux; viii, dimethyldioxirane, CH2Cl2, acetone, −78 °C→0 °C. | ||
 | ||
| Scheme 16 Reagents: i, t-BuOCl; ii, prenyltri(n-butyl)stannane, BCl3; iii, NH2NH2, MeOH, CH2Cl2; iv, N-Boc-Proline acid fluoride, CH2Cl2, NaHCO3, H2O; v, TMSI, MeCN, 0 °C; vi, NH3, MeOH. | ||
Two new cytotoxic epidithiodioxopiperadine dimers, 11,11′-dideoxyverticillin A 86a and 11′-deoxyverticillin A 86b together with known verticillin A, have been isolated from the mycelium of a marine-derived fungus of the genus Penicillium. A new monomeric bisdethio-bis(methylthio)-dioxopiperazine 87 have also been obtained by extraction of the culture broth. The structures and absolute stereochemistry have been assigned on the basis of NMR and CD spectral experiments. in vitro Cytotoxicity against HCT-116 human colon carcinoma of compounds 86a and 86b has been described (IC50 = 30 ng mL−1).68
The structure of arumdinine 92, isolated from the perennial giant gross Arundo donax, has recently been elucidated to be pyrrolidinoindoline skeleton having an ether bridge between the two indole units.71 In addition, the mass-spectrometric fragmentation of 92 has been investigated by high resolution mass spectrometry.72
Total synthesis of the pyrrolidinoindoline alkaloid debromoflustramine B 93, isolated from the marine bryozoan Flustra foliaceae together with closely related alkaloids,73 has been achieved by using the 2-oxofuro[2,3-b]indole 95 derived from the conjugated addition of the prenylmagnesium bromide to the 2-hydroxyindolenine 94 (Scheme 17). Related derivatives, debromoflustramine A and debromoflustramide A, possessing a reverse prenyl group at the 3a-position of hexahydropyrrolo[2,3-b]indole ring have also been synthesized.74
 | ||
| Scheme 17 Reagents: i, prenylmagnesium bromide, Et2O, THF; ii, Al2O3 (wet), THF, H2O; iii, MeONa, MeOH; iv, K2CO3, acetone, prenyl bromide; v, MeNH2, MeOH; vi, LiAlH4, THF. | ||
The enantiocontrolled total synthesis of (−)-chimonanthine 96, isolated from Idiospermum australiense,75 and the stereocontrolled total synthesis of meso-chimonanthine 97, isolated from Psychotria foresteriana,76 have been completed (Scheme 18).77 The first stereo- and enantioselective route for preparing chiral 3a,3a′-bis(pyrrolo[2,3-b]indolines) has been developed on the basis of a double Heck cyclization as the key step (98→99).
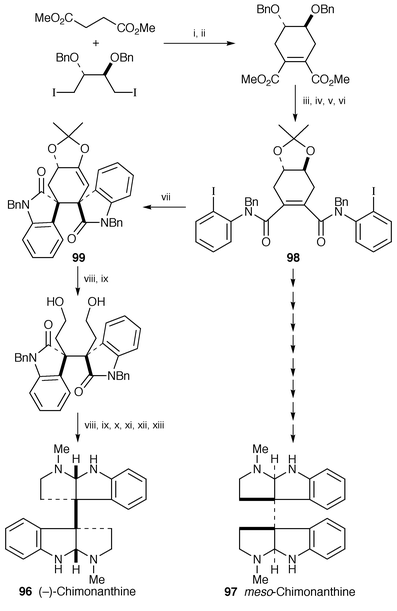 | ||
Scheme 18 Reagents: i, LDA, THF, HMPA; ii, LDA, THF, I2, −78 °C; iii, 2-iodoaniline, Me3Al, toluene, rt; iv, NaH, BuBr, DMF; v, BCl3, −78 °C; vi, 2,2-dimethoxypropane, camphorsulfonic acid (H2O); vii, 10% (PPh3)2PdCl2, Et3N, DMA, 100 °C; viii, Red-Al, THF, rt→reflux; ix, (PhO)2P(O)N3, PPh3, EtO2CN![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) NCO2Et, THF; x, H2, 10% Pd/C, EtOH; xi, MeOH, 110 °C; xii, HCHO, NaBH(OAc)3, MeOH; xiii, Na, NH3. NCO2Et, THF; x, H2, 10% Pd/C, EtOH; xi, MeOH, 110 °C; xii, HCHO, NaBH(OAc)3, MeOH; xiii, Na, NH3.
| ||
Total syntheses of marine alkaloids batzelline A 101a, batzelline B 101b, isobatzelline A 102a, and isobatzelline B 102b, isolated from the genus Batzella,79 have been reported. Their tricyclic pyrroloquinoline rings (103 and 104) have been prepared from a quinoline derivative (Scheme 19). Batzelline B 101b has also been synthesized from the pyrroloquinoline 104 in two steps.80
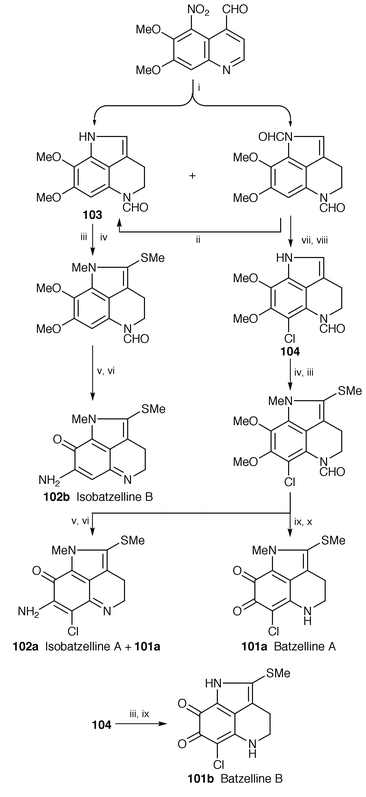 | ||
| Scheme 19 Reagents: i, NiCl2, NaBH4, MeOH, acetic formic anhydride; ii, DDQ, CH2Cl2, K2CO3, MeOH; iii, MeI, NaH, THF; iv, Me2S, SOCl2, CH2Cl2; v, aq NaOH, CAN, MeCN, H2O; vi, NH4Cl, MeOH, Ar, 40 °C; vii, NCS, DMF; viii, DDQ, CH2Cl2; ix, BBr3, CH2Cl2, O2; x, HCl, MeOH. | ||
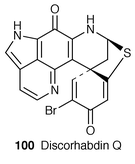
The first total synthesis of a new type of the pyrroloiminoquinone marine alkaloid veiutamine 105, isolated from the Fijian sponge Zyzzya fuliginosa,81 has been achieved (Scheme 20).82 The key step of this work is regioselective functionalization at the 6-position of pyrroloquinoline nucleus (106→107→108).83 This alkaloid has potent anticancer activity against solid tumors versus leukemia (IC50 = 0.12 μg mL−1 in a 25 cell line panel and IC50 = 0.3 μg mL−1 against the human colon tumor cell line HCT-116).81
 | ||
| Scheme 20 Reagents: i, t-BuLi, Et2O, 0 °C, Cl3CCCl3; ii, Ac2O, toluene, 0 °C; iii, Et2AlCN, toluene, 0 °C; iv, LiAlH4, benzene, Et2O, reflux; v, lithium isopropylcyclohexylamide, THF, 0 °C; vi, Boc2O, reflux; vii, s-BuLi, TMEDA, Et2O, −78 °C, p-MOMO-C6H4-CHO; viii, NaH, THF, 0 °C; ix, H2, Pd(OH)2-C, MeOH, AcOEt, rt; x, TBAF, THF, rt; xi, Fremy’s salt, pH = 7.0, 0 °C; xii, NH4Cl, MeOH, rt, cat. HCl, reflux, NaHCO3, TFA. | ||
A total synthesis of the marine alkaloid makaluvamine F 109, isolated from the Fijian sponge Zyzzya cf. marsailis and the Indonesian sponge Histodermella sp. (G),84 has been completed. The key steps are the formation of the pyrroloiminoquinone nucleus 110, the formation of the dihydrobenzothiophene ring 111, and the preparation of α-azidodihydrobenzothiophene 112 by the hypervalent iodine(III) induced reactions, respectively (Scheme 21).85 Makaluvamine F 109 exhibits the potent cytotoxicity towards the human colon tumor cell line HCT-116 (IC50 = 0.17 μM) and the inhibition of topoisomerase II.84
 | ||
Scheme 21 Reagents: i, PIFA-Me3SiOTf, (CF3)2CHOH, H2O; ii, PIFA, BF3·Et2O; iii, aq. MeNH2; iv, BF3·Et2O, EtSH; v, Ac2O; vi, PhI![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O, Me3SiN3; vii, NaOH, MeOH; viii, H2, 10% Pd/C, EtOH, TFA; ix, 110, MeOH, rt. O, Me3SiN3; vii, NaOH, MeOH; viii, H2, 10% Pd/C, EtOH, TFA; ix, 110, MeOH, rt.
| ||
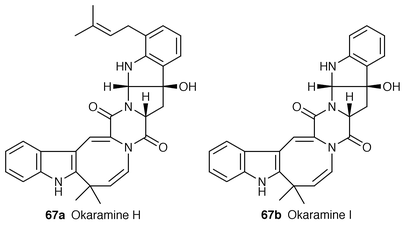
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-1,2,3,4-tetrahydromanzamine B 115 by CD spectra. This compound 115 exhibits cytotoxicity against L1210 murine leukemia cells and KB human epidermoid carcinoma cells (IC50 = 0.3 and 1.2 μg mL−1, respectively).88
)-1,2,3,4-tetrahydromanzamine B 115 by CD spectra. This compound 115 exhibits cytotoxicity against L1210 murine leukemia cells and KB human epidermoid carcinoma cells (IC50 = 0.3 and 1.2 μg mL−1, respectively).88Three new β-carboline alkaloids, (−)-isocyclocapitelline 116a, (+)-cyclocapitelline 116b and isocrysotricine 116c together with the known crysotricine (found in Hedyotis crysotricha![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 89), have been isolated from Hedyotis capitellata
89), have been isolated from Hedyotis capitellata![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 90 producing capitelline.91 Further, two new β-carboline alkaloids, hedyocapitelline 117a and hedyocapitine 117b, have been found from Hedyotis capitellata var. molis by the same group.92 Species of the genus Hedyotis have been used in the traditional Chinese and Vietnamese medicine.
90 producing capitelline.91 Further, two new β-carboline alkaloids, hedyocapitelline 117a and hedyocapitine 117b, have been found from Hedyotis capitellata var. molis by the same group.92 Species of the genus Hedyotis have been used in the traditional Chinese and Vietnamese medicine.
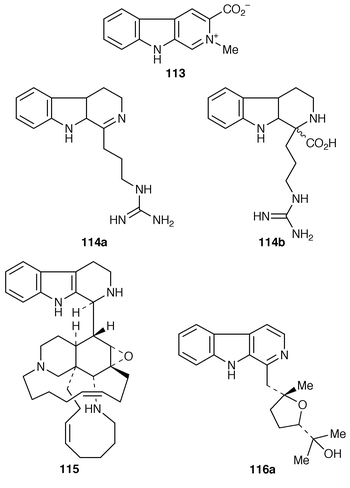
Isoharmine 118, isolated from the seeds of Perganum harmala,93 has been synthesized by a Pictet–Spengler reaction. Thermolysis of 3-cyanomethyl-2-vinylindole led to indolylacrylonitrile instead of 118.94 Total syntheses of N![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 9-substituted β-carboline alkaloids didemnolines A 119a, B 119b, C 120a and D 120b, isolated from the marine ascidian of the genous Didemum,95 have been accomplished. Didemnolines A 119a and B 119b have been synthesized by the reaction of β-carboline 121a or 121b with 4-chloromethylimidazole derivative 122. Didemnolines C 120a and D 120b have been derived by oxidation of A 119a and B 119b, respectively (Scheme 22).96
9-substituted β-carboline alkaloids didemnolines A 119a, B 119b, C 120a and D 120b, isolated from the marine ascidian of the genous Didemum,95 have been accomplished. Didemnolines A 119a and B 119b have been synthesized by the reaction of β-carboline 121a or 121b with 4-chloromethylimidazole derivative 122. Didemnolines C 120a and D 120b have been derived by oxidation of A 119a and B 119b, respectively (Scheme 22).96
 | ||
| Scheme 22 Reagents: i, KOH, DMF; ii, 6 M HCl, 110 °C; iii, NaIO4, MeOH, H2O, rt. | ||
Total syntheses of 4,8-dioxygenated β-carboline alkaloids 123a, 123b, 123c, and 123d, isolated from Simarubaceae plants,97 have been established.98 The key steps are an improved Fischer indolization![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 99 for the synthesis of 7-oxygenated indole 124 and the construction of a 4-methoxy-β-carboline skeleton 125 by an intramolecular C-3-selective cyclization (Scheme 23).
99 for the synthesis of 7-oxygenated indole 124 and the construction of a 4-methoxy-β-carboline skeleton 125 by an intramolecular C-3-selective cyclization (Scheme 23).
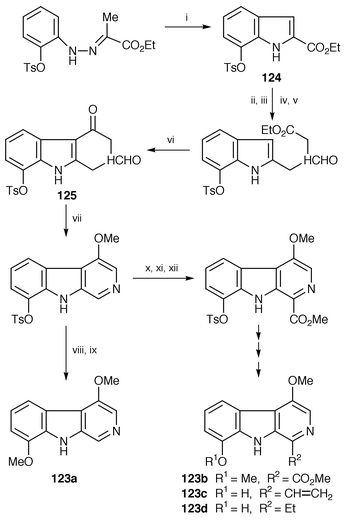 | ||
| Scheme 23 Reagents: i, Polyphosphoric acid, 70 °C; ii, DIBAL-H, CH2Cl2, −78 °C; iii, act MnO2, CH2Cl2; iv, NH2CH2CO2Et, NaBH3CN, MeOH, rt; v, HCO2Et, HCO2H, rt; vi, MsOH, 55 °C; vii, (MeO)2CMe2, TsOH, chloranil, benzene, rt; viii, Na-naphthalenide, 0 °C; ix, TMSCH2N2, THF, MeOH, rt; x, m-CPBA, CH2Cl2, rt; xi, diethylphosphoryl cyanide, Et3N, Cl(CH2)2Cl; xii, HCl, MeOH, reflux. | ||
Manzamine A 127, which exhibits potent antitumor activity in a number of assays,100 was the first member of this group of alkaloids to be isolated. Enantioselective total syntheses of a biogenetic precursor![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 101 of manzamine alkaloids ircinal A 126, isolated from the Okinawan marine sponge of Ircinia sp.102 and the formal total synthesis of manzamine A 127, isolated from the Okinawan marine sponge of the genera Haliclona sp. and Pellina sp.,100 have been completed. The key steps are a novel strategy for constructing the tricyclic ring core (128→129) by a domino Stille/Diels–Alder reaction and two sequential ring closing metathesis reactions by Grubbs ruthenium catalyst 130 for constructing 13- and 8-membered rings leading to ircinal A 126 (Scheme 24).103
101 of manzamine alkaloids ircinal A 126, isolated from the Okinawan marine sponge of Ircinia sp.102 and the formal total synthesis of manzamine A 127, isolated from the Okinawan marine sponge of the genera Haliclona sp. and Pellina sp.,100 have been completed. The key steps are a novel strategy for constructing the tricyclic ring core (128→129) by a domino Stille/Diels–Alder reaction and two sequential ring closing metathesis reactions by Grubbs ruthenium catalyst 130 for constructing 13- and 8-membered rings leading to ircinal A 126 (Scheme 24).103
 | ||
Scheme 24 Reagents: i, H2C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH-SnBu3, Pd(PPh3)4, toluene, reflux; ii, 0.13 equiv. 130, reflux; iii, KOH, MeOH, reflux; iv, H2C CH-SnBu3, Pd(PPh3)4, toluene, reflux; ii, 0.13 equiv. 130, reflux; iii, KOH, MeOH, reflux; iv, H2C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH(CH2)3COCl, Et3N; v, 1.1 equiv. 130, reflux, then 1 M HCl; vi, DIBAL-H; vii, Dess–Martin periodinane. CH(CH2)3COCl, Et3N; v, 1.1 equiv. 130, reflux, then 1 M HCl; vi, DIBAL-H; vii, Dess–Martin periodinane.
| ||
An approach to manzamine alkaloids based upon biogenetic considerations has been investigated by reactions of aminopentadienal derivatives with 5,6-dihydropyridinium salts.104
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 107 has been synthesized from harmalan in six-steps on the basis of a single electron transfer induced radical cationic hetero [4+2]cycloaddition (133→134) as the key step (Scheme 26).108
107 has been synthesized from harmalan in six-steps on the basis of a single electron transfer induced radical cationic hetero [4+2]cycloaddition (133→134) as the key step (Scheme 26).108 | ||
| Scheme 25 | ||
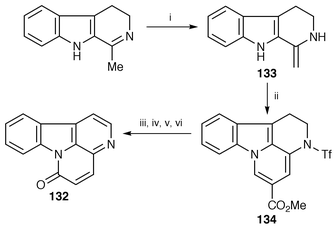 | ||
Scheme 26 Reagents: i, Tf2O, Et3N, CH2Cl2, 0 °C; ii, methyl (E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-3-(N,N-dimethylamino)acrylate, 400 mV, MeCN, CH2Cl2, 0.1 M LiClO4; iii, Na, naphthalene, DME, 0 °C; iv, MnO2, CH2Cl2; v, HCl; vi, Cu, pyridine. )-3-(N,N-dimethylamino)acrylate, 400 mV, MeCN, CH2Cl2, 0.1 M LiClO4; iii, Na, naphthalene, DME, 0 °C; iv, MnO2, CH2Cl2; v, HCl; vi, Cu, pyridine.
| ||
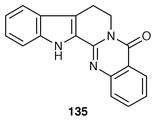
Three known indolopyridoquinazoline alkaloids have been isolated from the fruits of Evodia rutaecarpa by bioassay guided fractionation indicating with the inhibitory activity of cycloxygenase inhibitor (COX-2).109 A new COX-2 inhibitor has been identified as known rutaecarpine 135,110a which displays strong COX-2 inhibitory activity (IC50 = 80 ng mL−1). Two other alkaloids have also been identified as known related alkaloids, evodiamine and dehydroevodiamine.110b
3 Isoprenoid tryptamines
Biosynthesis of the pipecolate moiety of marcfortine A 136, isolated from the culture of Penicillium roqueforti,111 has been reported.112 It has been established that the pipecolic acid moiety of 136 is formed from L-lysine with![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 15N-labelled precursors.
15N-labelled precursors.A new teleocidin-related metabolite, (−)-14-O-malonylindolactam-V 137, has been isolated from the culture broth of Streptomyces blastmyceticum NA 34-17 which produces a large quantity of (−)-indolactam-V along with a small quantity of (−)-14-O-acetylindolactam-V.113
3.1 Ergot alkaloids
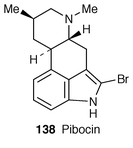
Pibocin 138, the first representative of marine ergoline alkaloids, has been found in the Far Eastern ascidian Eudistoma sp.114 Its structure and absolute stereochemistry have been established as (8β)-2-bromo-6,8-dimethylergoline on the basis of spectroscopic and X-ray analyses. Pibocin 138 exhibits cytotoxicity against mouse Ehlrich carcinoma cells (ED50 12.5 μg mL−1). The enantioselective separation method of the semisynthetic ergot alkaloids using the macrocyclic antibiotics, vancomycin and teicomycin as chiral stationary phase in reversed-phase HPLC, has been investigated.115The first total synthesis of optically active costaclavine 139, isolated from the culture of Agrppyrum or Penicillium type fungi,116 has been accomplished![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 117 starting from the tricyclic key compound 140 of chanoclavine synthesis.118 The key steps are the formation of the tricyclic ring 140 using palladium(0)-catalyzed intramolecular cyclization and the construction of the tetracyclic ring 141 having cis-conformation (Scheme 27).
117 starting from the tricyclic key compound 140 of chanoclavine synthesis.118 The key steps are the formation of the tricyclic ring 140 using palladium(0)-catalyzed intramolecular cyclization and the construction of the tetracyclic ring 141 having cis-conformation (Scheme 27).
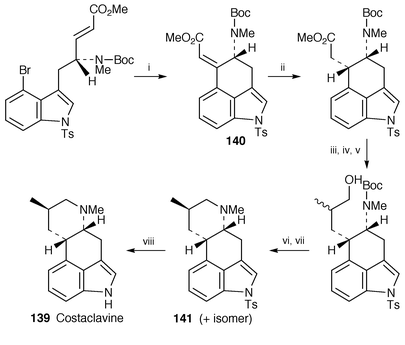 | ||
| Scheme 27 Reagents: i, Pd(0); ii, H2, Pd/C, MeOH, rt; iii, Tebbe reagent, toluene, THF, pyridine, −20 °C; iv, 10% HCl; v, BF3·THF, THF, 0 °C, then NaOH, H2O2; vi, TsCl, pyridine, DMAP, 50 °C; vii, TFA, CHCl3, rt; viii, Mg, MeOH, rt. | ||
4 Bisindole alkaloids
Phalarine 142 possessing a novel furanobisindole structure, has been isolated from Phalaris coerulescens.119 Its structure has been determined by mass and NMR spectrometric examinations. Three novel bromoindole sulfonic acids, echinosulfonic acids A 143a, B 143b, and C 143c along with a new sulfone, echinosulfone A 144 have been isolated from the marine sponge Echinodictyum sp. collected in Australia.120 The structures assigned to these compounds were on the basis of detailed spectroscopic analysis.Two new bisindole alkaloids bromodeoxytopsentin 145a and isobromodeoxytopsentin 145b have been isolated from the sponge Spongosorites genitrix together with known deoxytopsentin 145c and bromotopsentin 145d, and their structures have been elucidated by spectroscopic methods.121 These compounds exhibit moderate cytotoxicity against a human leukemia cell-line K-562 (IC50 = 0.6 and 2.1 μg mL−1, for 145a and 145b, respectively).
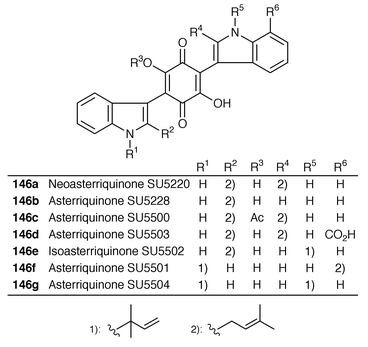
Five new asterriquinone analogs 146b, 146c, 146d, 146f and 146g, together with previously identified neoasterriquinone 146a and isoasterriquinone 146e, have been discovered in a fermentation broth of the fungs Aspergillus candidus.122 The structures have been determined by 1D and 2D NMR and MS/MS techniques. All seven compounds have shown inhibitory activity against the binding of the Grb-2 adapter to phosphorylated EGF receptor tyrosine kinase.
A new class of bisindole alkaloids iheyamine A 147a and B 147b, exhibiting moderate cytotoxicity, have been isolated from a colonial ascidian Polycitorella sp. collected off the Iheya island of Okinawa.123 Their structures have been established as a new polycyclic heteroaromatic system by interpretation of spectral data, but the absolute stereochemistry of iheyamine B 147b at C-1 and C-2 is still unknown.
The total synthesis of a bisindole alkaloid, hyrtiosin B 148 possessing cytotoxic activity against human epidermoid carcinoma KB cells, found in the Okinawan marine sponge Hyrtios erecta,124 has been achieved by a coupling reaction of the zinc metallated indole, prepared from 5-methoxyindole 149, with glyoxylyl chloride 150 as the key step (Scheme 28).125
 | ||
| Scheme 28 Reagents: i, (COCl)2, Et2O, 0 °C; ii, EtMgBr, Et2O, rt, ZnCl2, 150; iii, BBr3, CH2Cl2, −78 °C. | ||
The first total synthesis of a new bisindole alkaloid caulersin 151, isolated from the alga Caulerpa serrulata,126 has been achieved by employing a Michael type addition (152→153) and an intramolecular nucleophilic substitution (153→154) (Scheme 29).127
 | ||
| Scheme 29 Reagents: i, o-xylene, reflux; ii, methyl vinyl ketone, MeNO2, BF3·Et2O, 0 °C; iii, t-BuOK, t-BuOH, reflux; iv, DDQ, benzene, reflux; v, KOCl, MeOH, rt; vi 6 M HCl, MeOH, reflux. | ||
4.1 Indolocarbazoles
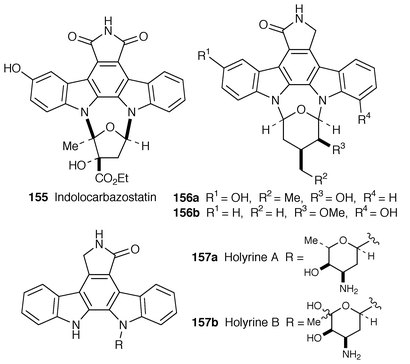
Indolocarbazostatin 155 has been isolated from a culture broth of Streptomyces sp.128 The compound 155 inhibits NGF-induced neurite outgrowth from PC12 cells at 6 nM, whereas K252a (158) inhibits at 200 nM under the same assay conditions. The IC50 values of 155 and K252a 158 for protein kinase C from rat brain are 2.0 and 25.0 nM, respectively. Two new indolocarbazole alkaloids, 3-hydroxy-3′-demethoxy-3′-hydroxystaurosporine 156a and 11-hydroxy-4′-N-demethylstaurosporine 156b together with five known staurosporine congeners have been isolated from the marine ascidian Eudistoma toealenis and its predatory flatworm Pseudoceros sp.129 The structures have been determined by 1D and 2D homonuclear NMR spectroscopies and from comparison with data of known compounds. A further two new indolocarbazole alkaloids holyrines A 157a and B 157b along with staurosporine, desmethylstaurosporine, and K252d have been isolated from the culture of marine actinomycete obtained from the North Atlantic Ocean,130 and their structures have been elucidated by spectroscopic analysis. Holyrines A 157a and B 157b are new members of the indolocarbazole alkaloids having a single attachment of amino sugar to the aromatic aglycon.The stereocontrolled total synthesis of indolocarbazole alkaloid (+)-K252a 158, isolated from Actinomadura sp. (SF-2370) and Nocardiopsis sp. K252a,131 has been accomplished in a twenty-three step sequence with an overall yield of 10%. The key steps are the regioselective N-glycosidation of the indole (159→161) and the construction of the indolo[2,3-a]carbazole framework by nonoxidative photocyclization (162→163) (Scheme 30).132
 | ||
| Scheme 30 Reagents: i, allyl bromide, K2CO3, DMF, rt; ii, NBS, CCl4, rt; iii, NaH, MeCN, 160, rt; iv, DBU, MS 4 Å, THF, 60 °C; v, i-Pr2NEt, hν, CH2Cl2, rt; vi, KI, I2, DBU, THF, rt. | ||
Indolocarbazole glycoside AT2433-B1 164, isolated from the cultures broth of Actinomadura melliaura ATCC39691![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 133 and Actinomadura melliaura SCC1655,134 has been synthesized by using Mannich cyclization (166→167) for the construction of indolo[2,3-a]carbazole system and the selective N-glycosidation (167→164) (Scheme 31).135 The stereochemistry of the amino sugar of 164 has also been revised by CD spectrum analysis.
133 and Actinomadura melliaura SCC1655,134 has been synthesized by using Mannich cyclization (166→167) for the construction of indolo[2,3-a]carbazole system and the selective N-glycosidation (167→164) (Scheme 31).135 The stereochemistry of the amino sugar of 164 has also been revised by CD spectrum analysis.
 | ||
| Scheme 31 Reagents: i, AgClO4, SnCl2, THF, −10 °C; ii, H2 (50 psi), Pd/C, DMF; iii, TFA (neat); iv, 165, MeOH, reflux; v, DDQ, THF; vi, HCl, MeOH. | ||
Improved synthesis of cytotoxic and antiviral 6-cyano-5-methoxy-12-methylindolo[2,3-a]carbazole 168, isolated from blue-green alga Nostoc sphaericum Ex-5-1,136 has been achieved starting from indigo 169 in six steps with an overall yield of 59% (Scheme 32).137
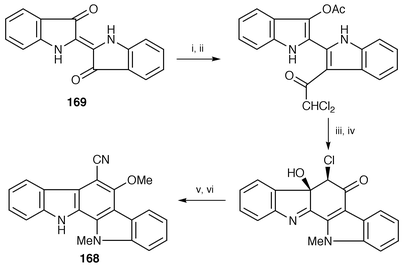 | ||
| Scheme 32 Reagents: i, Sn, AcOH, Ac2O, 64–65 °C; ii, Cl2CHCOCl, EtOAc, reflux; iii, aq. 1.3% NH3, MeOH, DMF, rt; iv, Me2SO4, K2CO3, rt; v, NaCN, DMF, H2O, 70 °C; vi, CH2N2, rt. | ||
4.2 Duocarmycins
CC-1065 and the duocarmycins are the parent members of a class of potent naturally occurring antitumor antibiotics which derive their biological properties through the sequence selective alkylation of duplex DNA. 1,2,9,9a-Tetrahydrocyclopenta[c]-benzoindol-4-one (CBI) analogs 170a and 170b incorporating the natural CPI alkylation subunit of CC-1065, have been synthesized and evaluated for their antitumor activity by Boger group.138 A new antibiotic gilvusmycin 171 has been isolated from the culture broth of Streptomyces sp. QM16.139 The structure has been related to CC-1065 and determined by NMR spectral analysis. Gilvusmycin 171 exhibits antitumor activity against murine leukemia P388 invivo.4.3 Dimeric β-carbolines
A total synthesis of a dimeric β-carboline alkaloid ochrolifuanine A 172, isolated from Apocynaceae,140 has been completed in seven steps by Michael type condensation of ethyl acrylate and tryptamine as the key step (Scheme 33).141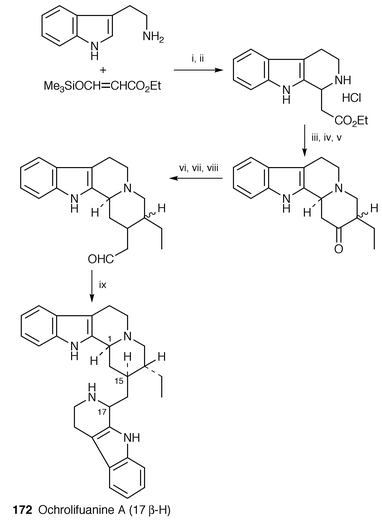 | ||
| Scheme 33 Reagents: i, THF, rt; ii, 10% HCl; iii, NaHCO3, H2O, EtOH, Et2O; iv, ethyl α-formylbutyrate, Et2O, reflux; v, NaH, benzene, reflux; vi, NaH, triethylphosphonoacetate, DME, rt; vii, H2, 10% Pd-C, EtOH, rt; viii, DIBAL-H, toluene, −73 °C; ix, tryptamine, 0.2 M phosphate buffer, 40 °C. | ||
5 Peptide alkaloids containing a tryptophan residue
A new antibiotic zelkovamycin 173 has been isolated from the fermentation broth of Streptomyces sp. K96-0670.142 Zelkovamycin 173 is a cyclic peptide containing a thiazole ring and a modified tryptophan residue, and the structure has been established by spectroscopic studies including 1H–1H COSY, 13C–1H COSY, 13C–1H HMQC, 13C–1H HMBC, 15N–1H HMQC and 15N–1H HMBC NMR experiments.Two jaspamide derivatives B 174a and C 174b along with jaspamide have been newly isolated from the marine sponge Jaspis splendans collected in Vanuatu.143 Their structures have been determined by 1D and 2D NMR experiments and MS data. These two compounds inhibit the growth of the NSCLC-N6 human tumor cell lines in vitro with IC50 values of 3.3 μg mL−1 and 1.1 μg mL−1, respectively.
A novel 14-membered cyclopeptide alkaloid Waltherine-C 175 has been isolated, together with four known cyclopeptide alkaloids from the bark of Waltheria douradinha (Sterculiaceae).144 The structure has been elucidated on the basis of 2D NMR spectral data, such as 1H–1H COSY, NOESY, HMQC and HMBC experiments, and FAB mass spectrum.
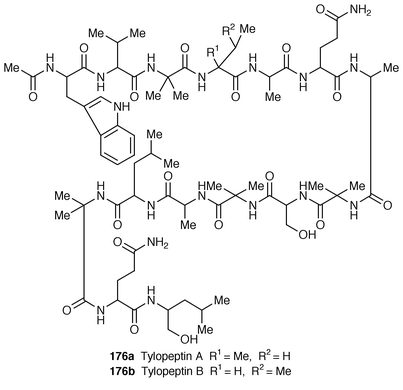
Two new peptides, tylopeptins A 176a and B 176b, have been isolated from the methanol extract of the fruiting body of the mushroom, Tylopilus neofelleus.145 Compounds 176a and 176b were determined to be linear peptides possessing an acetylated N-terminal residue, fourteen amino acids, and leucinol as C-terminal amino alcohol by FAB mass spectroscopy and two dimensional techniques (COSY and HOHAHA). These peptides exhibit antimicrobial activity against some Gram-positive bacteria.
Three new cyclic heptapeptides, cyclomarins A–C (177a–177c), have been isolated from the extract of a cultured marine bacterium collected in the vicinity of San Diego.146 The structure of the major component, cyclomarin A 177a contained three common and four unusual amino acids, and was determined using 1D and 2D NMR methods. Futhermore, the stereochemistry of the diacetate derivative of 177a has been determined by X-ray analysis. The structures of cyclomarins B 177b and C 177c have been assigned by comparison with spectral data for 177a. Cyclomarin A 177a shows antiinflammatory activity in both in vivo and in vitro assays.
The total synthesis of semi-synthetic cyclic heptapeptide dihydro-WIN67689 178c, isolated from Aspergillus flavipes![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 147 has been completed, and the stereochemistry of the β-hydroxy group of the isoprenyltyrosine moiety in 178a–c has been established as R-configuration (Scheme 34).148 Substance P antagonist activity of 178c is 70 times more potent than WIN66306 178a and the same potent as WIN67689 178b, isolated from the same species.
147 has been completed, and the stereochemistry of the β-hydroxy group of the isoprenyltyrosine moiety in 178a–c has been established as R-configuration (Scheme 34).148 Substance P antagonist activity of 178c is 70 times more potent than WIN66306 178a and the same potent as WIN67689 178b, isolated from the same species.
 | ||
Scheme 34 Reagents; i, (R)-Schöllkopf![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ’s reagent, BuLi, −78 to −20 °C; ii, 0.25 M TFA; iii, H2, Pd/C, MeOH; iv, Cbz-Gly-Val-Gly-OH, HOBt, EDCI, iPr2NEt, CH2Cl2; v, LiOH, MeCN; vi, TFA, Gly-Trp-Pro-Obn, HOBt, EDCI, iPr2NEt, CH2Cl2; vii, H2, Pd/C, MeOH; viii, pentafluorophenyl diphenylphosphinate, DMF, iPr2NEt. ’s reagent, BuLi, −78 to −20 °C; ii, 0.25 M TFA; iii, H2, Pd/C, MeOH; iv, Cbz-Gly-Val-Gly-OH, HOBt, EDCI, iPr2NEt, CH2Cl2; v, LiOH, MeCN; vi, TFA, Gly-Trp-Pro-Obn, HOBt, EDCI, iPr2NEt, CH2Cl2; vii, H2, Pd/C, MeOH; viii, pentafluorophenyl diphenylphosphinate, DMF, iPr2NEt.
| ||
The first total synthesis of cyclic heptadepsipeptide HUN-7293 180, isolated from a fungal broth during a screen for potent inhibitors of inducible cell adhesion molecule expression and from a different fungal species based on a screen for anti-HIV compounds,149 has been achieved by the Boger group.150 The approach is based on a Mitsunobu esterification between tetrapeptide 181 and tripeptide 182 followed by macrocyclization (183→180) (Scheme 35). It has been confirmed by biological evaluation that synthetic HUN-7293 180 is indistinguishable from the naturally occurring cyclodepsipeptide.
 | ||
| Scheme 35 Reagents; i, Ph3P, DIAD, toluene; ii, TFA, anisole; iii, HATU, collidine. | ||
The first total synthesis of keramamide J 184, isolated from an Okinawan marine sponge Theonella sp.,151 has been established.152 The synthesis has been achieved by using four fragment amides 185, 186, 187 and 190 which are assembled from the appropriate amino acid constituents (Scheme 36). The structure of synthetic keramamide J 184 was not identical with that of natural keramamide J.
 | ||
| Scheme 36 Reagents; i, HCl, Et2O; ii, DCC, HOBt, iPr2NEt; iii, Pd(PPh3)4, dimedone; iv, DCC, HOBt, iPr2NEt; v, Pd(PPh3)4, dimedone; vi, Et2NH; vii, (PhO)2PON3, NaHCO3; viii, HCl, MeOH; ix, HATU, 2,4,6-collidine; x, o-iodoxybenzoic acid, DMSO; xi, Amberlite IR-120. | ||
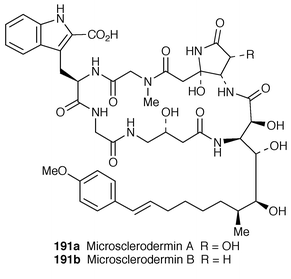
A synthetic approach to a 23-membered cyclic hexapeptide, microsclerodermines A 191a and B 191b, isolated from the lithistid marine sponge Microscleroderma sp.,153 has been reported by Shioiri group.154
6 Indole alkaloids with a nonrearranged monoterpenoid unit
Three new alkaloids, 3-hydroxywelwitindolinone C isonitrile 192a, 3-hydroxy-N-methylwelwitindolinone C isothiocyanate 192b, and N-methylwelwitindolinone D isonitrile 192d together with 3-hydroxy-N-methylwelwitindolinone C formamide 192c and known welwitindolinones![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 155 have been isolated from two terrestrial Fischerella spp. belonging to the Stigonemataceae.156 The compound 192a is readily converted to 3-hydroxy-N-methylwelwitindolinone C formamide 192c by hydration during the isolation procedure. The compound 192d is a novel cyclic ether of welwitindolinones.
155 have been isolated from two terrestrial Fischerella spp. belonging to the Stigonemataceae.156 The compound 192a is readily converted to 3-hydroxy-N-methylwelwitindolinone C formamide 192c by hydration during the isolation procedure. The compound 192d is a novel cyclic ether of welwitindolinones.References
- (a) D. J. Faulkner, Nat. Prod. Rep., 2000, 17, 1 RSC; (b) D. J. Faulkner, Nat. Prod. Rep., 2000, 17, 7 RSC.
- J. R. Lewis, Nat. Prod. Rep., 2000, 17, 57 RSC.
- M. Nakagawa, Y. Torisawa, H. Uchida and A. Nishida, J. Synth. Org. Chem. Jpn., 1999, 57, 1002 Search PubMed.
- A. Qureshi and D. J. Faulkner, J. Nat. Prod., 1999, 13, 59 CAS.
- I. Çalis, A. Kuruüzüm and P. Rüedi, Phytochemistry, 1999, 50, 1205 CrossRef CAS.
- L. Suescun, R. A. Mariezcurrena, A. W. Mombrú, D. Davyt and E. Monta, Acta Crystallogr., Sect. A, Fundam. Crystallogr., 1999, 55, 211 CrossRef.
- N. Phay, T. Higashiyama, M. Tsuji, H. Matsuura, Y. Fukushi, A. Yokota and F. Tomita, Phytochemistry, 1999, 52, 271 CrossRef CAS.
- T. R. Kelly, Y. Fu and R. L. Xie, Tetrahedron Lett., 1999, 40, 1857 CrossRef.
- (a) Y. Koyama, R. Yokose and L. J. Dolby, J. Agric. Biol. Chem., 1981, 45, 1285 Search PubMed; (b) M. Noltemeyer, G. M. Sheldrick, H.-U. Hoppe and A. Zeeck, J. Antibiot., 1982, 35, 549 Search PubMed; (c) K. Umehara, K. Yoshida, M. Okamoto, M. Iwami, H. Tanaka, M. Kohsaka and H. Imanaka, J. Antibiot., 1984, 37, 1153 Search PubMed; (d) D. S. Bhate, R. K. Hulyalkar and S. K. Menon, Experientia, 1966, 16, 504 Search PubMed; (e) B. S. Joshi, W. I. Taylor, D. S. Bhate and S. S. Karmarkar, Tetrahedron, 1963, 19, 1437 CrossRef CAS.
- L. Garlaschelli, Z. Pang, O. Sterner and G. Vidari, Tetrahedron, 1994, 50, 3541.
- B. C. Söderberg, A. G. Chisnell, S. N. O’Neil and J. A. Shriver, J. Org. Chem., 1999, 64, 9731 CrossRef.
- G. B. Quistad, C. C. Reuter, W. S. Skinner, P. A. Dennis, S. Suwanrumpha and E. W. Fu, Toxicon, 1991, 29, 329 CrossRef CAS.
- Y. Hida, T. Kan and T. Fukuyama, Tetrahedron Lett., 1999, 40, 4711 CrossRef.
- S. P. Gunasekera, P. J. McCarthy, R. E. Longley, S. A. Pomponi and A. E. Wright, J. Nat. Prod., 1999, 62, 1208 CrossRef CAS.
- O. D. Hensens, J. G. Ondeyka, A. W. Dombrowski, D. A. Ostlind and D. L. Zink, Tetrahedron Lett., 1999, 40, 5455 CrossRef CAS.
- J. G. Ondeyka, G. L. Helms, O. D. Hensens, M. A. Goetz, D. L. Zink, A. Tsipouras, W. L. Shoop, L. Slayton, A. W. Dombrowski, J. D. Polishook, D. A. Ostlind, N. N. Tsou, R. G. Ball and S. B. Singh, J. Am. Chem. Soc., 1997, 119, 8809 CrossRef CAS.
- J. Hurter, T. Ramp, B. Patrian, E. Städler, P. Roessingh, R. Baur, R. de Jong, J. K. Niersen, T. Winkler, W. J. Richter, D. Müller and B. Ernst, Phytochemistry, 1999, 51, 377 CrossRef CAS.
- M. S. C. Pedras, J. L. Sorensen, F. I. Okanga and I. L. Zaharia, Bioorg. Med. Chem. Lett., 1999, 9, 3015 CrossRef CAS.
- (a) S. J. Danishefsky and J. M. Schkeryantz, Synlett, 1995, 475 CrossRef CAS; (b) Z. Wang and L. S. Jimenez, Tetrahedron Lett., 1996, 37, 6049 CrossRef CASand related references cited therein..
- S. Lee, W.-M. Lee and G. A. Sulikowski, J. Org. Chem., 1999, 64, 4224 CrossRef CAS.
- W. Dong and L. S. Jimenez, J. Org. Chem., 1999, 64, 2520 CrossRef CAS.
- M. T. H. Nutan, C. M. Hasan and M. A. Rashid, Fitoterapia, 1999, 70, 130 CrossRef CAS.
- T.-S. Wu, S.-C. Huang, P.-L. Wu and C.-S. Kuoh, Phytochemistry, 1999, 52, 523 CrossRef CAS.
- A. K. Chakravarty, T. Sarkar, K. Masuda and K. Shiojima, Phytochemistry, 1999, 50, 1263 CrossRef CAS.
- G. Lin and A. Zhang, Tetrahedron Lett., 1999, 40, 341 CrossRef CAS.
- M. Kaneda, T. Naid, T. Kitahara, S. Nakamura, T. Hirata and T. Suga, J. Antibiot., 1988, 41, 602 Search PubMed and related references cited therein..
- H.-J. Knölker and W. Fröhner, Tetrahedron Lett., 1999, 40, 6915 CrossRef CAS.
- J. H. Cardellina II, M. P. Kirkup, R. E. Moore, J. S. Mynderse, K. Seff and C. J. Simmons, Tetrahedron Lett., 1979, 4915 CrossRef.
- H.-J. Knölker, E. Baum and T. Hopfmann, Tetrahedron, 1999, 55, 10391 CrossRef CAS.
- C. Ito and H. Furukawa, Chem. Pharm. Bull., 1990, 38, 1548 Search PubMed.
- T. Soós, G. Timári and G. Hajós, Tetrahedron Lett., 1999, 40, 8607 CrossRef CAS.
- (a) H. W. Rauwald, M. Kobar, E. Mutschler and G. Lambrecht, Planta Med., 1992, 58, 486 CAS; (b) K. Cimanga, T. De Bruyne, A. Lasure, B. Van Poel, L. Pieters, M. Claeys, D. Vanden Berghe, K. Kambu, L. Tona and A. J. Vlietinck, Planta Med., 1996, 62, 22 CAS; (c) K. Cimanga, T. De Bruyne, L. Pieters and A. J. Vlietinck, J. Nat. Prod., 1997, 60, 688 CrossRef CAS; (d) D. E. Bierer, D. M. Fort, C. D. Mendez, J. Lou, P. A. Imbach, L. G. Dubenko, S. D. Jolad, R. E. Gerber, J. Litvak, Q. Lu, P. Zhang, M.-J. Reed, N. Waldeck, R. C. Bruening, B. K. Noamesi, R. F. Hector, T. J. Carlson and S. R. King, J. Med. Chem., 1998, 41, 894 CrossRef CAS.
- C. E. Hadden, M. H. M. Sharaf, J. E. Guido, R. H. Robins, A. N. Tackie, C. H. Phoebe Jr., P. L. Schiff Jr. and G. E. Martin, J. Nat. Prod., 1999, 62, 238 CrossRef CAS.
- (a) J. L. Pousset, A. Jossag and B. Bock, Phytochemistry, 1995, 39, 735 CrossRef CAS; (b) K. Cimanga, T. De Bruyne, L. Pieters, M. Claeys and A. Vlietinck, Tetrahedron Lett., 1996, 37, 1703 CrossRef CAS; (c) M. H. M. Sharaf, P. L. Schiff Jr., A. N. Tackie, C. H. Phoebe Jr. and G. E. Martin, J. Heterocycl. Chem., 1996, 33, 239 Search PubMed.
- P. M. Fresneda, P. Molina and S. Delgado, Tetrahedron Lett., 1999, 40, 7275 CrossRef CAS.
- P. Molina, P. M. Fresneda and S. Delgado, Synthesis, 1999, 326 CrossRef CAS.
- E. Arzel, P. Rocca, F. Marsais, A. Godard and G. Quéguiner, Tetrahedron, 1999, 55, 12149 CrossRef CAS.
- (a) E. Arzel, P. Rocca, F. Marsais, A. Godard and G. Quéguiner, Tetrahedron Lett., 1998, 39, 6465 CrossRef CAS; (b) P. Rocca, C. Cochennec, F. Marsais, L. Thomas-dit-Dumont, M. Mallet, A. Godard and G. Quéguiner, J. Org. Chem., 1993, 58, 7832 CrossRef CAS.
- C.-Y. Chen, F.-R. Chang, C.-M. Teng and Y.-C. Wu, J. Chin. Chem. Soc., 1999, 46, 77 Search PubMed.
- D. Chávez, L. A. Acevedo and R. Mata, J. Nat. Prod., 1999, 62, 1119 CrossRef CAS.
- Y. Kamano, H. Morita, R. Takano, A. Kotake, T. Nogawa, H. Hashima, K. Takeya, H. Itokawa and G. R. Pettit, Heterocycles, 1999, 50, 499 Search PubMed.
- N. M. Cuong, W. C. Taylor and T. V. Sung, Phytochemistry, 1999, 52, 1711 CrossRef CAS.
- (a) A. Hofmann, R. Heim and H. Kobel, Experientia, 1958, 14, 107 Search PubMed; (b) A. Hofmann, R. Heim, A. Brack, H. Kobel, A Frey, H. Ott, T. Petrzilka and F. Troxler, Helv. Chim. Acta, 1959, 42, 1557 CrossRef CAS; (c) T. J. Petcher and H. P. Weber, J. Chem. Soc., Perkin Trans. 2, 1974, 946 RSC.
- H. Sakagami and K. Ogasawara, Heterocycles, 1999, 51, 1131 Search PubMed.
- H. Takayama, T. Shimizu, H. Sada, Y. Harada, M. Kitajima and N. Aimi, Tetrahedron, 1999, 55, 6841 CrossRef CAS.
- (a) Y. Kamano, H. Zhang, Y. Ichihara, H. Kizu, K. Komiyama and G. R. Pettit, Tetrahedron Lett., 1995, 36, 2783 CrossRef CAS; (b) H. Zhang, Y. Kamano, Y. Ichihara, H. Kizu, K. Komiyama, H. Itokawa and G. R. Pettit, Tetrahedron, 1995, 51, 5523 CrossRef CAS.
-
(a) G. K. Jnaneshwar and V. H. Deshpande, J. Chem. Res. (S
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ), 1999, 632 Search PubMed;
(b) G. K. Jnaneshwar, A. V. Bedekar and V. H. Deshpande, Synth. Commun., 1999, 29, 3627.
), 1999, 632 Search PubMed;
(b) G. K. Jnaneshwar, A. V. Bedekar and V. H. Deshpande, Synth. Commun., 1999, 29, 3627. - D. J. Hart and N. Magomedov, Tetrahedron Lett., 1999, 40, 5429 CrossRef CAS.
- A. Jossang, P. Jossang, H. A. Hadi, T. Sevenet and B. Bodo, J. Org. Chem., 1991, 56, 6527 CrossRef CAS.
- G. Lakshmaiah, T. Kawabata, M. Shang and K. Fuji, J. Org. Chem., 1999, 64, 1699 CrossRef CAS.
- J. H. Rigby and J. H. Meyer, Synlett, 1999, 860 CAS.
- H. C. Vevoort, W. Fenical and P. A. Keifer, J. Nat. Prod., 1999, 62, 389 CrossRef CAS.
- R. G. S. Berlinck, R. Britton, E. Piers, L. Lim, M. Roberge, R. M. da Rocha and R. J. Andersen, J. Org. Chem., 1998, 63, 9850 CrossRef CAS.
- D. Sørensen. T. O. Larsen, C. Cristophersen, P. H. Nielsen and U. Anthoni, Phytochemistry, 1999, 51, 1181 CrossRef.
- H. Fujimoto, T. Fujimaki, E. Okuyama and M. Yamazaki, Chem. Pharm. Bull., 1999, 47, 1426 Search PubMed.
- H. Hayashi, K. Furutsuka and Y. Shiono, J. Nat. Prod., 1999, 62, 315 CrossRef CAS.
- Y. Shiono, K. Akiyama and H. Hayashi, Biosci. Biotechnol. Biochem., 1999, 63, 1910 Search PubMed.
- J. E. Hochlowski, M. M. Mullaly, S. G. Spanton, D. N. Whittern, P. Hill and J. B. McAlpine, J. Antibiot., 1993, 46, 380 Search PubMed.
- (a) S. Takase, M. Iwami, T. Ando, M. Okamoto, K. Yoshida, H. Horiai, M. Kohsaka, H. Aoki and H. Imanaka, J. Antibiot., 1984, 37, 1320 Search PubMed; (b) S. Takase, Y. Kawai, I. Uchida, H. Tanaka and H. Aoki, Tetrahedron, 1985, 41, 3037 CrossRef CAS.
- K. M. Depew, S. P. Marsden, D. Zatorska, A. Zatorski, W. G. Bornmann and S. J. Danishefsky, J. Am. Chem. Soc., 1999, 121, 11953 CrossRef CAS.
- (a) C.-B. Cui, H. Kakeya, G. Okada, R. Onose and H. Osada, J. Antibiot., 1996, 49, 527 Search PubMed; (b) C.-B. Cui, H. Kakeya and H. Osada, Tetrahedron, 1997, 53, 59 CrossRef CAS; (c) T. Usui, M. Kondoh, C.-B. Cui, T. Mayumi and H. Osada, Biochem. J., 1998, 333, 543 CAS.
- (a) S. Edmondson, S. J. Danishefsky, L. Sepp-Lorenzino and N. Rosen, J. Am. Chem. Soc., 1999, 121, 2147 CrossRef; (b) S. Edmondson and S. J. Danishefsky, Angew. Chem., Int. Ed., 1998, 37, 1138 CrossRef CAS.
- (a) C. Shinohara, K. Hasumi, Y. Takei and A. Ando, J. Antibiot., 1994, 47, 163 Search PubMed; (b) B. Nuber, F. Hansske, C. Shinohara, S. Miura, K. Hasumi and A. Ando, J. Antibiot., 1994, 47, 168 Search PubMed.
- (a) J. M. Schkeryantz, J. C. G. Woo, P. Siliphaivanh, K. M. Depew and S. J. Danishefsky, J. Am. Chem. Soc., 1999, 121, 11964 CrossRef CAS; (b) K. M. Depew, S. J. Danishefsky, N. Rosen and L. Sepp-Lorenzino, J. Am. Chem. Soc., 1996, 118, 12463 CrossRef CAS.
- (a) P. S. Steyn, Tetrahedron Lett., 1971, 3331 CrossRef CAS; (b) P. S. Steyn, Tetrahedron, 1973, 29, 107 CrossRef CAS.
- (a) A. J. Birch and J. J. Wright, J. Chem. Soc., Chem. Commun., 1969, 644 RSC; (b) A. J. Birch and J. J. Wright, Tetrahedron, 1970, 26, 2329 CrossRef CAS.
- (a) C.-B. Cui, H. Kakeya, G. Okada, R. Onose, M. Ubukata, I. Takahashi, K. Isono and H. Osada, J. Antibiot., 1995, 48, 1382 Search PubMed; (b) C.-B. Cui, H. Kakeya and H. Osada, J. Antibiot., 1996, 49, 534 Search PubMed.
- B. W. Son, P. R. Jensen, C. A. Kauffman and W. Fenical, Nat. Prod. Lett., 1999, 13, 213 Search PubMed.
- L. Verotta, F. Peterlongo, E. Elisabetsky, T. T. Amador and D. S. Nunes, J. Chromatogr. A, 1999, 841, 165 CrossRef CAS.
- V. Jannic, F. Guéritte, O. Laprévote, L. Serani and M.-T. Martin, J. Nat. Prod., 1999, 62, 838 CrossRef CAS.
- I. Zhalolov, V. U. Khuzhaev, B. Tashkhodzhaev, M. G. Levkovich, S. F. Aripova and N. D. Abdullaev, Chem. Nat. Compd., 1998, 34, 706 Search PubMed.
- I. Zhalolov, V. U. Khuzhaev, U. A. Abdullaev and S. F. Aripova, Chem. Nat. Compd., 1999, 35, 221 Search PubMed.
- C. Christophersen, Acta Chem. Scand., Ser. B, 1985, 39, 517 Search PubMed.
- M. S. Morales-Ríos, O. R. Suárez-Castille and P. Joseph-Nathan, J. Org. Chem., 1999, 64, 1086 CrossRef CAS.
- R. K. Duke, R. D. Allan, G. A. R. Johnston, K. N. Mewett, A. D. Mitrovic, C. C. Duke and T. W. Hambley, J. Nat. Prod., 1995, 58, 1200 CrossRef CAS.
- Y. Adjibade, B. Weniger, J. C. Quirion, B. Kuballa, P. Cabalion and R. Anton, Phytochemistry, 1992, 31, 317 CrossRef CAS.
- L. E. Overman, D. V. Paone and B. A. Stearns, J. Am. Chem. Soc., 1999, 121, 7702 CrossRef CAS.
- M.-G. Dijoux, W. R. Gamble, Y. F. Hallock, J. H. Cardellina II, R. van Soest and M. R. Boyd, J. Nat. Prod., 1999, 62, 636 CrossRef.
- (a) H. H. Sun, S. Sakemi, N. Burres and P. McCarthy, J. Org. Chem., 1990, 55, 4964 CrossRef CAS; (b) S. Sakemi, H. H. Sun, C. W. Jefford and G. Bernadinelli, Tetrahedron Lett., 1989, 30, 2517 CrossRef CAS.
- (a) M. Alvarez, M. A. Bros and J. A. Joule, Eur. J. Org. Chem., 1999, 1173 CrossRef CAS; (b) M. Alvarez, M. A. Bros and J. A. Joule, Tetrahedron Lett., 1998, 39, 679 CrossRef CAS.
- D. A. Vanables, L. R. Barrows, P. Lassota and C. M. Ireland, Tetrahedron Lett., 1997, 38, 721 CrossRef.
- Y. Moro-oka, T. Fukuda and M. Iwao, Tetrahedron Lett., 1999, 40, 1713 CrossRef CAS.
- (a) M. Iwao, O. Motoi, T. Fukuda and F. Ishibashi, Tetrahedron, 1998, 54, 8999 CrossRef CAS; (b) W. F. Bailey and S. C. Longstaff, J. Org. Chem., 1998, 63, 432 CrossRef CAS.
- (a) D. C. Radisky, E. S. Radisky, L. R. Barrows, B. R. Copp, R. A. Kramer and C. M. Ireland, J. Am. Chem. Soc., 1993, 115, 1632 CrossRef CAS; (b) L. R. Barrows, D. C. Radisky, B. R. Copp, D. S. Swaffer, R. A. Kramer, R. L. Warters and C. M. Ireland, Anti-Cancer Drug Des., 1993, 8, 333 Search PubMed.
- (a) Y. Kita, M. Egi, T. Takada and H. Tohma, Synthesis, 1999, 885 CrossRef CAS; (b) Y. Kita, M. Egi and H. Tohma, Chem. Commun., 1999, 143 RSC.
- G. M. Cabrera and A. M. Seldes, J. Nat. Prod., 1999, 62, 759 CrossRef CAS.
- R. M. Van Wagoner, J. Jompa, A. Tahir and C. M. Ireland, J. Nat. Prod., 1999, 62, 794 CrossRef CAS.
- M. Tsuda, D. Watanabe and J. Kobayashi, Heterocycles, 1999, 50, 485 Search PubMed.
- J. N. Peng, X. Z. Feng, Q. T. Zheng and X. T. Liang, Phytochemistry, 1997, 46, 1119 CrossRef.
- N. M. Phuang, T. V. Sung, A. Porzel, J. Schmidt, K. Merzweiler and G. Adam, Phytochemistry, 1999, 52, 1725 CrossRef.
- N. M. Phuang, T. V. Sung, J. Schmidt, A. Porzel and G. Adam, Nat. Prod. Lett., 1998, 11, 93 Search PubMed.
- N. M. Phuang, T. V. Sung, A. Porzel, J. Schmidt and G. Adam, Planta Med., 1999, 65, 761.
- M. T. Ayoub and L. J. Rashan, Phytochemistry, 1991, 30, 1046 CrossRef CAS.
- J. Sapi, D. Patigny and J.-Y. Lorenze, Heterocycles, 1999, 51, 361 Search PubMed.
- R. W. Schumacher and B. S. Davidson, Tetrahedron, 1995, 51, 10125 CrossRef CAS.
- R. W. Schumacher and B. S. Davidson, Tetrahedron, 1999, 55, 935 CrossRef CAS.
- (a) T. Ohmoto, K. Koike, T. Higuchi and K. Ikeda, Chem. Pharm. Bull., 1985, 33, 3356 Search PubMed; (b) T. Ohmoto and K. Koike, Chem. Pharm. Bull., 1985, 33, 3847 Search PubMed; (c) C. Souleles and E. Kokkalou, Planta Med., 1989, 55, 286 CASand related references cited therein..
- H. Suzuki, M. Unemoto, M. Hagiwara, T. Ohyama, Y. Yokoyama and Y. Murakami, J. Chem. Soc., Perkin Trans. 1, 1999, 1717 RSC.
- Y. Murakami, Y. Yokoyama, C. Aoki, H. Suzuki, K. Sakurai, T. Shinohara, C. Miyagi, Y. Kimura, T. Takahashi, T. Watanabe and T. Ohmoto, Chem. Pharm. Bull., 1991, 39, 2189 Search PubMed.
- (a) R. Sakai, T. Higa, C. W. Jefford and G. Bernardinelli, J. Am. Chem. Soc., 1986, 108, 6404 CrossRef CAS; (b) H. Nakamura, S. Deng, J. Kobayashi, Y. Ohizumu, Y. Tomotake, T. Matsuzaki and Y. Hirata, Tetrahedron Lett., 1987, 28, 621 CrossRef CAS; (c) N. Matzanke, R. Gregg and S. Weinreb, Org. Prep. Proc. Int., 1998, 30, 1 Search PubMed; (d) E. Magnier and Y. Langlois, Tetrahedron, 1998, 54, 6201 CrossRef CAS.
- (a) J. E. Baldwin and R. C. Whitehead, Tetrahedron Lett., 1992, 33, 2059 CrossRef CAS; (b) J. E. Baldwin, T. D. W. Claridge, A. J. Culshaw, F. A. Heupel, V. Lee, D. R. Spring, R. C. Whitehead, R. J. Boughtflower, I. M. Mutton and R. J. Upton, Angew. Chem., Int. Ed., 1998, 37, 2661 CrossRef CAS; (c) M. Tsuda and J. Kobayashi, Heterocycles, 1997, 46, 765 Search PubMed.
- K. Kondo, H. Shigemori, Y. Kikuchi, M. Ishibashi, T. Sasaki and J. Kobayashi, J. Org. Chem., 1992, 57, 2480 CrossRef CAS.
- S. F. Martin, J. M. Humphrey, A. Ali and M. C. Hillier, J. Am. Chem. Soc., 1999, 121, 866 CrossRef CAS.
- K. Jakubowicz, K. B. Abdeljelil, M. Herdemann, M.-T. Martin, A. Gateau-Olesker, A. A. Mourabit, C. Marazano and B. C. Das, J. Org. Chem., 1999, 64, 7381 CrossRef.
- (a) T. Ohmoto and K. Koike, Chem. Pharm. Bull., 1982, 30, 1204 Search PubMed; (b) T. Ohmoto and K. Koike, Chem. Pharm. Bull., 1985, 33, 3847 Search PubMed; (c) T. Ohmoto and K. Koike, Chem. Pharm. Bull., 1985, 33, 4901 Search PubMed; (d) K. Matsunaga, K. Koike, T. Sawaguchi and T. Ohmoto, Phytochemistry, 1994, 35, 799 CrossRef.
- K. Koike, H. Yoshino and T. Nikaido, Heterocycles, 1999, 51, 315 Search PubMed.
- (a) T. Ohmoto, R. Tanaka and T. Nikaido, Chem. Pharm. Bull., 1976, 24, 1532 Search PubMed; (b) T. Ohmoto, K. Koike and Y. Sakamoto, Chem. Pharm. Bull., 1981, 29, 390 Search PubMed; (c) T. Ohmoto and K. Koike, Chem. Pharm. Bull., 1984, 32, 170 Search PubMed; (d) H. F. Haynes, E. R. Nelson and J. R. Price, Aust. J. Sci. Res., 1952, 5, 385 Search PubMed; (e) J.-H. Li and J. K. Snyder, Tetrahedron Lett., 1994, 35, 1485 CrossRef CAS.
- U. Rößler, S. Blechert and E. Steckhan, Tetrahedron Lett., 1999, 40, 7075 CrossRef.
- S. S. Kang, J. S. Kim, K. H. Son. H. P. Kim and H. W. Chang, Nat. Prod. Sci., 1999, 5, 65 Search PubMed.
- (a) H. Itokawa, M. Inamatsu and K. Takeya, Shoyakugaku Zasshi, 1990, 44, 135 Search PubMed; (b) A. Haji, Y. Momose, R. Takeda, S. Nakanishi, T. Horiuch and M. Arisawa, J. Nat. Prod., 1994, 57, 387 CrossRef CAS.
- J. Polonsky, M.-A. Merrien, T. Prange and C. J. Pascard, J. Chem. Soc., Chem. Commun., 1980, 601 RSC.
- M. S. Kuo, D. A. Yurek, S. A. Mizsak, J. I. Cialdella, L. Baczynskyj and V. P. Marshall, J. Am. Chem. Soc., 1999, 121, 1763 CrossRef CAS.
- K. Irie, S. Tomimatsu, Y. Nakagawa, H. Ohigashi and H. Hayashi, Biosci. Biotechnol. Biochem., 1999, 63, 1669 Search PubMed.
- T. N. Makarieva, S. G. Ilyin, V. A. Stonik, K. A. Lyssenko and V. A. Denisenko, Tetrahedron Lett., 1999, 40, 1591 CrossRef CAS.
- E. Tesarová, K. Záruba and M. Flieger, J. Chromatogr. A, 1999, 844, 137 CrossRef CAS.
- (a) M. Abe, S. Yamatodani, T. Yamano and M. Kusumoto, Bull. Agric. Chem. Soc. Jpn., 1956, 20, 59 Search PubMed; (b) S. Yamatodani and M. Abe, J. Agric. Chem. Soc. Jpn., 1960, 34, 366 Search PubMed.
- K. Osanai, Y. Yokoyama, K. Kondo and Y. Murakami, Chem. Pharm. Bull., 1999, 47, 1587 Search PubMed.
- Y. Yokoyama, K. Kondo, M. Mitsuhashi and Y. Murakami, Tetrahedron Lett., 1996, 37, 9309 CrossRef CAS.
- N. Anderton, P. A. Cockrum, S. M. Colegate, J. A. Edgar, K. Flower, D. Gardner and R. I. Willing, Phytochemistry, 1999, 51, 153 CrossRef CAS.
- S. P. B. Ovenden and R. J. Capon, J. Nat. Prod., 1999, 62, 1246 CrossRef CAS.
- J. Shin, Y. Seo, K. W. Cho, J.-R. Rho and C. J. Sim, J. Nat. Prod., 1999, 62, 647 CrossRef CAS.
- K. A. Alvi, H. Pu, M. Luche, A. Rice, H. App, H. Dare and B. Margolis, J. Antibiot., 1999, 52, 215 Search PubMed.
- T. Sasaki, I. I. Ohtani, J. Tanaka and T. Higa, Tetrahedron Lett., 1999, 40, 303 CrossRef CAS.
- J. Kobayashi, T. Murayama, M. Ishibashi, S. Kosuge, M. Takamatsu, Y. Ohizumi, H. Kobayashi, T. Ohta, S. Nozoe and T. Sasaki, Tetrahedron, 1990, 46, 7699 CrossRef CAS.
- J. Bergman, T. Janosik and A.-L. Johnsson, Synthesis, 1999, 580 CrossRef CAS.
- J.-Y. Su, Y. Zhu, L.-M. Zeng and X.-H. Xu, J. Nat. Prod., 1997, 66, 1043 CrossRef CAS.
- P. M. Fresneda, P. Molina and M. A. Saez, Synlett, 1999, 1651 CAS.
- M. Ubukata, N. Tamehiro, N. Matsuura and N. Nakajima, J. Antibiot., 1999, 52, 921 Search PubMed.
- P. Schupp, C. Eder, P. Proksch, V. Wray, B. Schneider, M. Herderich and V. Paul, J. Nat. Prod., 1999, 62, 959 CrossRef CAS.
- D. E. Williams, V. S. Bernan, F. V. Ritacco, W. M. Maiese, M. Greenstein and R. J. Andersen, Tetrahedron Lett., 1999, 40, 7171 CrossRef CAS.
- (a) M. Sezaki, T. Sasaki, T. Nakazawa, U. Takeda, M. Iwata, T. Watanabe, M. Koyama, F. Kai, T. Shomura and M. Kojima, J. Antibiot., 1985, 38, 1439 Search PubMed; (b) H. Kase, K. Iwahashi and Y. Matsuda, J. Antibiot., 1986, 39, 1059 Search PubMed.
- Y. Kobayashi, T. Fujimoto and T. Fukuyama, J. Am. Chem. Soc., 1999, 121, 6501 CrossRef CAS.
- J. Golik, T. W. Doyle, B. Krishnan, G. Dubay and J. A. Matson, J. Antibiot., 1989, 42, 1784 Search PubMed.
- J. A. Matson, C. Claridge, J. A. Bush, J. Titus, W. T. Bradner, T. W. Doyle, A. C. Horan and M. Patel, J. Antibiot., 1989, 42, 1547 Search PubMed.
- J. D. Chisholm, J. Golik, B. Krishnan, J. A. Matson and D. L. Van Vranken, J. Am. Chem. Soc., 1999, 121, 3801 CrossRef CAS.
- G. Knübel, L. K. Larsen, R. E. Moore, I. A. Levine and G. M. L. Patterson, J. Antibiot., 1990, 43, 1236 Search PubMed.
- H. Hayashi, Y. Suzuki and M. Somei, Heterocycles, 1999, 51, 1233 Search PubMed.
- D. L. Boger, C. W. Boyce, R. M. Garbaccio, M. Searcey and Q. Jin, Synthesis, 1999, 1505 CrossRef CAS.
- Y. Tokoro, T. Isoe and K. Shindo, J. Antibiot., 1999, 52, 263 Search PubMed.
- N. Peube-Locou, M. Koch, M. Plat and P. Potier, C. R. Acad. Sci., 1971, 905, 273 Search PubMed.
- I. P. Malhotra and N. Malhotra, J. Serb. Chem. Soc., 1999, 64, 155 Search PubMed.
- H. Zhang, H. Tomoda, N. Tabata, M. Oohori, M. Shinose, Y. Takahashi and S. Omura, J. Antibiot., 1999, 52, 29 Search PubMed.
- A. Zampella, C. Giannini, C. Debitus, C. Roussakis and M. V. D’Auria, J. Nat. Prod., 1999, 62, 332 CrossRef CAS.
- A. F. Morel, A. Flach, N. Zanatta, E. M. Ethur, M. A. Mostardeiro and I. T. S. Gehrke, Tetrahedron Lett., 1999, 40, 9205 CrossRef CAS.
- S.-J. Lee, B.-S. Yun, D.-H. Cho and I.-D. Yoo, J. Antibiot., 1999, 52, 998 Search PubMed.
- M. K. Renner, Y.-C. Shen, X.-C. Cheng, P. R. Jensen, W. Frankmoelle, C. A. Kauffman, W. Fenical, E. Lobkovsky and J. Clardy, J. Am. Chem. Soc., 1999, 121, 11273 CrossRef CAS.
- (a) C. J. Barrow, M. S. Doleman, M. A. Bobko and R. Cooper, J. Med. Chem., 1994, 37, 356 CrossRef CAS; (b) C. J. Barrow, D. M. Sedlock, H. H. Sun, R. Cooper and A. M. Gillum, J. Antibiot., 1994, 47, 1182 Search PubMed.
- Y.-Q. Li, K. Sugase and M. Ishiguro, Tetrahedron Lett., 1999, 40, 9097 CrossRef CAS.
- (a) U. Hommel, H.-P. Weber, L. Oberer, H. U. Naegeli, B. Oberhauser and C. A. Foster, FEBS Lett., 1996, 379, 69 CrossRef CAS; (b) H. Itazaki, T. Fujiwara, A. Sato, Y. Kawamura and K. Matsumoto, Jpn Pat., Kokai Tokkyo Koho 07109299, 1995, Appl. 93/255592, 13 Oct. 1993; Chem. Abstr., 1995, 123, 110270 Search PubMed.
- D. L. Boger, H. Keim, B. Oberhauser, E. P. Shreiner and C. A. Foster, J. Am. Chem. Soc., 1999, 121, 6197 CrossRef CAS.
- J. Kobayashi, F. Itagaki, H. Shigemori, T. Takao and Y. Shimonishi, Tetrahedron, 1995, 51, 2525 CrossRef CAS.
- J. A. Sowinski and P. L. Toogood, Chem. Commun., 1999, 981 RSC.
- C. A. Bewley, C. Debitus and D. J. Faulkner, J. Am. Chem. Soc., 1994, 116, 7631 CrossRef CAS.
- S. Sasaki, Y. Hamada and T. Shioiri, Tetrahedron Lett., 1999, 40, 3187 CrossRef CAS.
- (a) K. Startmann, R. E. Moore, R. Bonjouklian, J. B. Decter, G. M. Patterson, S. Shaffer, C. D. Smith and T. A. Smitka, J. Am. Chem. Soc., 1994, 116, 9935 CrossRef; (b) D. Klein, D. Daloze, J. C. Braekman, L. Hoffmann and V. Demoulin, J. Nat. Prod., 1995, 58, 1781 CrossRef CAS.
- J. I. Jimenez, U. Huber, R. E. Moore and G. M. L. Patterson, J. Nat. Prod., 1999, 62, 569 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2001 |