Amaryllidaceae, Sceletium, imidazole, oxazole, thiazole, peptide and miscellaneous alkaloids
John R. Lewis
Department of Chemistry, University of Aberdeen, Meston Walk, Old Aberdeen, UK AB24 3UE
First published on 19th December 2000
Abstract
Covering: July 1998–June 1999. Previous review: 2000, 17, 57.
1 Introduction
Amaryllidaceae alkaloids continue to be reported and to contribute to clinical investigations. This year no muscarine alkaloids were reported but imidazole, oxazole and thiazole alkaloids continue to contribute novel structural arrangements. Peptides are ever of interest and in some cases are included in sections other than Section 4 where a more dominant heterocyclic system is thought to be a priority.2 Amaryllidaceae and Sceletium alkaloids
The mucilage secreted by the bulbs of Narcissus tazetta contains an inhibitor towards the germination of rice and Chinese cabbage seedlings and to their growth.1 The active compound was found to be narciclasine 1. Lycorine 2 has been found to have an inhibitory effect on tumour cell apoptosis which is induced by polymorphonuclear leukocyte-derived calprotection.2Nine genera of the Mesembryanthemaceae family have been investigated for alkaloid content by gas chromatography followed by nitrogen-phosphorous detection. Sceletium tortuosum contained three alkaloids, 4′-O-methylmesembrenol 3, mesembrine 4, and mesembrenone 5. When this plant is processed through fermentation a reportedly psychoactive homeopathic preparation called kougoed is produced. This fermentation procedure has been repeated and the product shows a substantial increase in alkaloid content compared to just drying the plant material at 80 °C prior to extraction.3
An oxidative conversion of (−)-galanthamine 6 into lycoraminone 7 can be achieved in one step![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 4 using potassium hydride in toluene in the presence of hexamethylphosphoramide at 60 °C. The yield is 85%.
4 using potassium hydride in toluene in the presence of hexamethylphosphoramide at 60 °C. The yield is 85%.
A patent describing the resolution of (±)-narwedine 8 indicates that it can be induced by seeding the racemic solution with (−)-narwedine provided an amine is also added. The solvent to amine ratio needs to be greater than 15![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ∶
∶![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 1; thus 8 gave a 99.1% ee in ethanol.5 Crystals of homolycorine hydrochloride dihydrate can be obtained when the alkaloid 9 is dissolved in an aqueous solution containing HCl and the water allowed to evaporate.6 An X-ray analysis of these crystals indicates unambiguously the chirality of the molecule which is the same as earlier measurements and opposite to that of o-methyllycorenine hydrochloride.
1; thus 8 gave a 99.1% ee in ethanol.5 Crystals of homolycorine hydrochloride dihydrate can be obtained when the alkaloid 9 is dissolved in an aqueous solution containing HCl and the water allowed to evaporate.6 An X-ray analysis of these crystals indicates unambiguously the chirality of the molecule which is the same as earlier measurements and opposite to that of o-methyllycorenine hydrochloride.
The dried bulbs of Crinum amabile, from Vietnam, have been shown to contain seven alkaloids. Two are new, namely crinamabine 10 and 4α-dehydroxycrinamabine 11. The others were lycorine 2, buphanisine 12, augustine 13, while ambelline 14 and flexinine 15 are reported for the first time as metabolites in this species.7 An investigation into the petroleum ether extract of the bulbs of Crinum augustum Rox has shown![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 8 ungeremine 16 to be the only alkaloid present. Six Vietnamese Crinum species have been examined, namely C. amabile, C. asiaticum, C. defixum, C. giganteum, C. latifolium and C. moorei. Data for more than fifteen alkaloids were presented.9 The fresh bulbs of South African grown Crinum delagoense contain six alkaloids, the four known ones are lycorine 2, hamayne 17, 6-hydroxycrinamine 18 and criwelline 19, while delagoensine 20 and delagoenine 21 are new.10 An X-ray crystallographic analysis of (−)-crinine 22 has established that the N-containing five-membered ring and the D-ring have envelope conformations, while A and B rings have distorted chair and half-chair conformations respectively.11Crinum stuhlmannii found in Kenya contains 9-O-demethylpluvine 23, kirkine 24, ambelline 14, crinine 22 and hamayne 17.12
8 ungeremine 16 to be the only alkaloid present. Six Vietnamese Crinum species have been examined, namely C. amabile, C. asiaticum, C. defixum, C. giganteum, C. latifolium and C. moorei. Data for more than fifteen alkaloids were presented.9 The fresh bulbs of South African grown Crinum delagoense contain six alkaloids, the four known ones are lycorine 2, hamayne 17, 6-hydroxycrinamine 18 and criwelline 19, while delagoensine 20 and delagoenine 21 are new.10 An X-ray crystallographic analysis of (−)-crinine 22 has established that the N-containing five-membered ring and the D-ring have envelope conformations, while A and B rings have distorted chair and half-chair conformations respectively.11Crinum stuhlmannii found in Kenya contains 9-O-demethylpluvine 23, kirkine 24, ambelline 14, crinine 22 and hamayne 17.12
Four alkaloids, isolated from two Galanthus species, are allocated to a novel sub group of the Amaryllidaceae alkaloid family. Extraction of the dried, powdered, total plant Galanthus gracilis produced two alkaloids (+)-graciline 25 and the bisalkaloid (−)-digracine 26 while Galanthus plicatus also contained 26, the 11-acetoxy-derivative 27 and (+)-3,4-dihydro-3-hydroxygraciline 28.13 Unfortunately the choice of the trivial stem name graciline will lead to confusion as it is already used in the literature for non-alkaloids. A sub-species of one of these Turkish Amaryllidaceae Galanthus plicatus subspecies byzantinus, upon investigation, has produced two new related alkaloids namely (+)-plicamine 29 and (−)-secoplicamine 30. Both alkaloids have a new skeletal arrangement compared to other Amaryllidaceae alkaloids and it is suggested that members of this sub-group be called plicamines.14 Another publication on the constituents of Galanthus plicatus subspecies byzantinus indicated that (+)-tazettine 31, and (+)-3-epimacronine 33 were constituents while (+)-3-O-demethylcriwelline 32 was a constituent of Galanthus gracilis. All these alkaloids had their structures confirmed by X-ray analysis.15Hymenocallis tubiflora from the West Indies![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 16 contains lycorine 2, pseudolycorine 34 and pretazzetine 35.
16 contains lycorine 2, pseudolycorine 34 and pretazzetine 35.
A review of the chemistry and pharmacology of Narcissus alkaloids, with 312 references, has been published.17 An examination of whole plants of Narcissus bujei has revealed the presence of eleven alkaloids, namely homolycorine 36, 8-O-demethylhomolycorine 37, masonine 38, lycorenine 39, O-methyllycorenine 40, O-methyloduline 41, crinamine 42, haemanthamine 43, tazettine 31 and the new ones are 11-O-acetylhaemanthamine 44 and bujeine 45. This publication![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 18 also reexamines the chirality of O-methyllycorenine 40 by X-ray diffraction. Using the hydrochloride salt it was established that its chirality was opposite to that previously proposed for this alkaloid. In the tissue culturing of mature seeds of Narcissus confusus it has been found that different calli produced galanthamine 6 in different amounts. This suggests that culture tissue differentiation has to play a big part in future plant culture procedures. Interestingly, after two years, these calli all regenerated plants with normal morphological characteristics.19
18 also reexamines the chirality of O-methyllycorenine 40 by X-ray diffraction. Using the hydrochloride salt it was established that its chirality was opposite to that previously proposed for this alkaloid. In the tissue culturing of mature seeds of Narcissus confusus it has been found that different calli produced galanthamine 6 in different amounts. This suggests that culture tissue differentiation has to play a big part in future plant culture procedures. Interestingly, after two years, these calli all regenerated plants with normal morphological characteristics.19
Pancratium maritium bulbs grown in Turkey have yielded twelve alkaloids. (−)-N-Demethylgalanthamine 46 and (+)-tazettine 31 were fully characterised as they had not been found in this plant species before.20 While flowers of an Egyptian plant have produced lycorine 2, maritidine 47, lycoramine 48 and galanthamine 6.21 The air dried bulbs of Zephyranthes carinata have produced two new alkaloids belonging to the pancratistatin group.22 One is 1-O-(3-hydroxybutyryl)pancratistatin 49 and the other 1-O-(3-O-D-D-glucopyranosylbutyryl)pancratistatin 50.
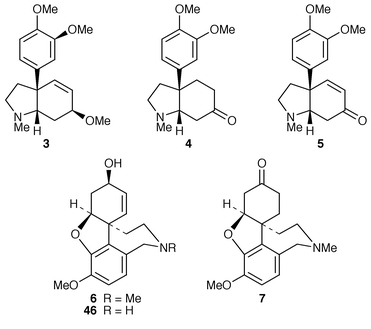
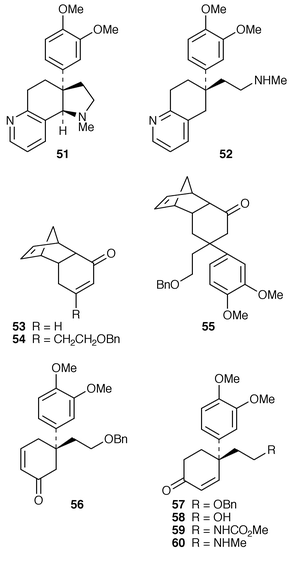
The absolute configuration of the Sceletium alkaloids (−)-mesembrine 4, (+)-sceletium A-4 51 and (+)-tortuosamine 52 has been established by a stereocontrolled synthesis of (+)-sceletium A-4.23 The synthesis was initiated with the tricyclic enone 53 which could be alkylated in the β-position in a single flask sequential Michael–Wittig procedure giving 54. 3,4-Dimethoxyphenylmagnesium bromide reacted with 54 to give the β,β-disubstituted ketone 55 after acidic work up. Thermolysis gave enone 56 which was converted into its isomer 57. Deprotection and conversion of the alcohol 58 into carbamate 59 and thence amine 60 thus allowed cyclisation to give (−)-mesembrine 4. Through a modification to enone 57 it was possible to synthesise (+)-sceletium A-4 51 and (−)-tortuosamine 52. These three alkaloids have also been synthesised by a different asymmetric route.24 In this case allyl alcohol 61 was converted into bromoacetal 62 which on treatment with NaBH4 in the presence of Bu3SnCl and AIBN gave furan 63. Modification of the furan ring gave amine 64 which led to enone 65 which cyclised to (−)-mesembrine 4. The other two alkaloids were also produced by adaptation of this asymmetric pathway.24
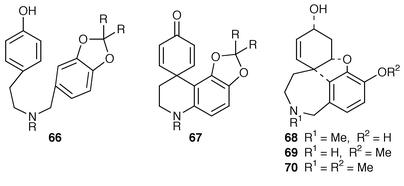
A patent utilising hypervalent iodine reagents has been described whereby galanthamine type alkaloids can be prepared.25 Typically benzylamine 66 upon treatment with iodine(III) reagents, e.g. PhI(OCOCF3)2 in CF3CH2OH at −40 °C for 1.5 hours, gave dienone 67 which could be quantitatively converted into (±)-narwedine 8. This synthetic procedure has been more fully described in chemical terms by the same group.26 In this publication phenyliodine(III) reagents were used to synthesise (±)-sanguinine 68, (±)-norgalanthamine 69 and (±)-lycoramine 70.
(±)-γ-Lycorine 71 has been synthesised through the radical cyclisation of 72 with copper(I) and (II) chlorides![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 27 thus giving a mixture of chlorides 73. Iodine exchange with NaI in acetone produced only one diiodo product 74 due to the inability of the α-chloride to react. Elimination of HI (75) was followed by radical cyclisation (Bu3SnH, AlBN) to create the natural racemic alkaloid 71.
27 thus giving a mixture of chlorides 73. Iodine exchange with NaI in acetone produced only one diiodo product 74 due to the inability of the α-chloride to react. Elimination of HI (75) was followed by radical cyclisation (Bu3SnH, AlBN) to create the natural racemic alkaloid 71.
(+)-7-Deoxypancratistatin 76 has been synthesised by a much improved radical based process. In the original synthesis![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 28 lactone 77 could not be cyclised via78 in the normal radical procedure because the use of Bu3SnH caused lactone carbonyl reduction. This radical type cyclisation can now be achieved using Ph3SnH
28 lactone 77 could not be cyclised via78 in the normal radical procedure because the use of Bu3SnH caused lactone carbonyl reduction. This radical type cyclisation can now be achieved using Ph3SnH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 29 whereby the radical cyclisation is carried out on an ether 79 which cyclised to 80. Conversion of protecting group OBn into COCF3 followed by introduction of the carbonyl group with PCC gave 81. Removal of the protecting group with BF3–diethyl ether thus allowed SmI2 cleavage of the N–O bond with the resulting lactam being converted into lactone (+)-7-deoxypancratistatin 76. The second synthetic method in this area describes the use of a β-azidonation reaction to create (+)-pancratistatin 82 itself.30 Essentially chiral 83 was converted into azide 84 using iodosylbenzene with TMSN3 at −15 °C in CH2Cl2. This reagent must, according to the authors, not be older than three months (freezer stored). Azide 84 was then converted into the trans-amide 85 and thence through standard procedures to the natural product 82.
29 whereby the radical cyclisation is carried out on an ether 79 which cyclised to 80. Conversion of protecting group OBn into COCF3 followed by introduction of the carbonyl group with PCC gave 81. Removal of the protecting group with BF3–diethyl ether thus allowed SmI2 cleavage of the N–O bond with the resulting lactam being converted into lactone (+)-7-deoxypancratistatin 76. The second synthetic method in this area describes the use of a β-azidonation reaction to create (+)-pancratistatin 82 itself.30 Essentially chiral 83 was converted into azide 84 using iodosylbenzene with TMSN3 at −15 °C in CH2Cl2. This reagent must, according to the authors, not be older than three months (freezer stored). Azide 84 was then converted into the trans-amide 85 and thence through standard procedures to the natural product 82.
A synthesis of (+)-coccinine 86 has been achieved through an azaallyl anion cycloaddition procedure involving 87 and 88. The result is an enantioselective product 89 which on formylation, deprotection and elimination gave (+)-coccinine 86, the enantiomer of the natural occurring alkaloid.31 The synthesis of NK109 an anticancer benzo[c]phenanthridine alkaloid 90 has been reported.32 Condensation of aldehyde 91 with naphthylamine 92 gave bromoamine 93. This was followed by intramolecular radical cyclisation using AIBN in the presence of tributyltin hydride; yields of up to 90% were obtained of the cyclised product 90.
In a fully versatile synthetic procedure eight benzo[c]phenanthridine alkaloids have been synthesised.33 Basically a 2-bromonaphthylamine 94 was added, by Suzuki coupling, to a 2-formylboronic acid 95 in toluene–DME with toluene-p-sulfonic acid at reflux. The resulting amine, upon formylation, underwent Bischler–Napieralski cyclisation to give the tetracyclic product 96 and thence to the alkaloid series 97.
A total synthesis of chelerythrine 98 and nitidine 99 has been carried out successfully using a novel palladium–phosphine procedure.34 This reagent, prepared from Pd(OMe)2, DPPP, i.e. 1,3-bis(diphenylphosphino)propane, and Bu3P, enabled an aryl to aryl coupling to take place utilising precursors such as 100, where X can be triflate or halide.
3 Imidazole, oxazole and thiazole alkaloids
A synthesis of almazole A 101 and almazole B 102 has been achieved in seven steps starting from a protected phenylalanine.35 The procedure was accomplished, without racemization, using N-(tetrachlorophthaloyl)phenylalanine as its acid chloride 103 to couple with isoxazolone 104 to give isoxazole 105. Irradiation rearranged it to oxazole 106 whence deprotection with hydrazine allowed the amine 107 to be methylated by reductive formylation. The N,N-dimethyl derivative 108 reacted with 109 to give almazole A 101 which upon deformylation produced almazole B 102.The sea alga Martensia fragilis produces a potent inhibitor of lipid peroxidation. The active metabolite has been identified as martefragin A 110 and its first synthesis has been reported starting with R-citronellol![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 36111, (Scheme 1). Removal of the hydroxy group was followed by oxidative cleavage of the double bond to produce carboxylic acid 112 which was condensed (via its acid chloride) with oxazolidine 113 to give 114. Asymmetric azidation using the Evans procedure gave azide 115 in diastereomerically pure form. Conversion of this azide into the protected (2S,4S)-homoisoleucine 116 occurred in three steps whence it was condensed with O-benzyl-L-tryptophan hydrochloride to give dipeptide 117. This dipeptide cyclised upon treatment with DDQ to give ozazole 118. N,N-Dimethylation and debenzylation resulted in (1S,3S)-martefragin A 110. The spectral properties of the synthetic product were in good agreement with those of the natural product thus indicating that martefragin A has either the 1S,3S or 1R,3R absolute configuration.
36111, (Scheme 1). Removal of the hydroxy group was followed by oxidative cleavage of the double bond to produce carboxylic acid 112 which was condensed (via its acid chloride) with oxazolidine 113 to give 114. Asymmetric azidation using the Evans procedure gave azide 115 in diastereomerically pure form. Conversion of this azide into the protected (2S,4S)-homoisoleucine 116 occurred in three steps whence it was condensed with O-benzyl-L-tryptophan hydrochloride to give dipeptide 117. This dipeptide cyclised upon treatment with DDQ to give ozazole 118. N,N-Dimethylation and debenzylation resulted in (1S,3S)-martefragin A 110. The spectral properties of the synthetic product were in good agreement with those of the natural product thus indicating that martefragin A has either the 1S,3S or 1R,3R absolute configuration.
 | ||
| Scheme 1 Reagents and conditions: (i) MsCl, Et3N; (ii) LiAlH4, Et2O; (iii) KMnO4, NaIO4 aq acetone; (iv) SOCl2, bz; (v) Li-(S)-4-benzyl-2-oxo-1,3-oxazolidine; (vi) KN(TMS)2, THF, −78 °C then 2,4,6-triisopropylbenzenesulfonyl azide, −78 °C then HOAc, −78 °C; (vii) LiOH, aq THF; (viii) H2, 10% Pd/C, EtOH; (ix) B(Boc)2O, NaOH, aq dioxane; (x) benzyl-L-tryptophan HCl, diethyl phosphonocyanidate, Et3N, THF, 0 °C then rt; (xi) DDQ, THF, reflux; (xii) CF3CO2H, CH2Cl2; (xiii) formalin, AcOH dioxane, H2, 10% Pd/C; (xiv) H2, Pd/C, AcOEt. | ||
B-90063 119 is a novel endothelin-converting-enzyme inhibitor from a new marine bacterium called Blastobacter; SANK 71894 was the species allocation.37
Phorboxazole A 120 and its C13 epimer have an unprecedented array of oxane, oxazole, macrolide and polyene moieties. Its synthesis has been reported![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 38 through the assembly of three synthons, all synthesised in previous publications. To begin oxane 121
38 through the assembly of three synthons, all synthesised in previous publications. To begin oxane 121![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 39 was coupled with a second oxane containing synthon 122
39 was coupled with a second oxane containing synthon 122![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 40 to give 123. Dess–Martin periodinane oxidation gave oxazole 124 which could be converted into the diethylphosphonoacetate C-3 aldehyde 125. Rapid cyclisation occurred under Masamune–Roush conditions but the ratio of Z
40 to give 123. Dess–Martin periodinane oxidation gave oxazole 124 which could be converted into the diethylphosphonoacetate C-3 aldehyde 125. Rapid cyclisation occurred under Masamune–Roush conditions but the ratio of Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ∶
∶![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) E acrylate was predominately to the unrequired E. Treatment of 125 with K2CO3 and 18-crown-6 ether gave a 4
E acrylate was predominately to the unrequired E. Treatment of 125 with K2CO3 and 18-crown-6 ether gave a 4![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ∶
∶![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 1 ratio of Z to E and this ratio could not be improved upon. The removal of the acetonide protecting group now enabled the third, previously synthesised,41 synthon 126 to be added, giving 127. Formation of the second oxazole ring (Dess–Martin procedure) led to the natural antitumour active product 120.
1 ratio of Z to E and this ratio could not be improved upon. The removal of the acetonide protecting group now enabled the third, previously synthesised,41 synthon 126 to be added, giving 127. Formation of the second oxazole ring (Dess–Martin procedure) led to the natural antitumour active product 120.
Three new mycalolides have been isolated from the marine sponge Mycale magellanica,42 all are derived from mycalolide A 128 or mycalolide B 129 which was also present in the extract. 30-Hydroxymycalolide 130 has a hydroxy group at C30, 32-hydroxymycaloloide is 131 and 38-hydroxymycaloloide 132 has its hydroxy group at C38.
Clavosines A, B and C are potent cytotoxins as well as being inhibitors of protein phosphatases 1 and 2A. The marine sponge Myriastra clavosa is the source of these three metabolites, which have structures 133, 134 and 135 respectively.43
In a new analysis of the secondary metabolites of the sponge Aplysina couliformis, obtained from the Caribbean, eight compounds were identified, six were known, but two are new. All are bromo-metabolites related to tyrosine. The new ones, being unnamed, were designated 13 and 14 and are 136 and 137 respectively.44
The macrocyclic thiopeptide promothiocin A has been synthesised for the first time![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 45 by assembling three components in the order shown in structure 138.
45 by assembling three components in the order shown in structure 138.
The total synthesis of cyclodidemnamide 139 has resulted in a revision of its previous structure. The valine section of the macrocyclic ring being the D not L stereoisomer.46
The total synthesis of raocyclamide A 140 was achieved through cyclisation of raocyclamide B 141 with the Burgess reagent. Raocyclamide B 141 was synthesised through the successive condensation of thiazole 142 and thence with D-Phe-D-Ser-methyl ester to give peptide 143 which with oxazole 144 gave tripeptide 145. Deprotection followed by treatment of the amino-acid with Hunig’s base under high dilution conditions gave raocyclamide B 141 which thence produced raocyclamide A 140. This synthesis![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 47 revises the previous structure for these natural products whereby L-isoleucine was the stereoisomer involved not D-isoleucine as previously thought.
47 revises the previous structure for these natural products whereby L-isoleucine was the stereoisomer involved not D-isoleucine as previously thought.
Fungerin 146 is a metabolite produced by a fungus of the Fusarium family found growing in a stalk of Miscanthus sacchariflorus. Its synthesis has been accomplished by first prenylating diiodo-N-methylimidazole 147 to give 148 which under Heck conditions gave fungerin 146 in high yield.48
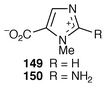
Three novel betains have been isolated from the marine sponge Agelas dispar.49 Two are pyridine-derived and the other is a derivative of zooanemonin 149, namely aminozooanemonin 150.
Didemnimides A, B, C and D have been found in the Caribbean mangrove ascidian Didemnum conchyliatum. These indole–maleimide–imidazole tricyclic fused alkaloidal systems are thought to be predator deterrants. The synthesis of didemnimide A 151 and B 152 have been achieved utilising a new method for the synthesis of unsymmetrical bis(indolyl)maleimides.50 Thus methyl indolyl-3-glyoxylate 153 or its 6-bromo-counterpart 154 was condensed with acetamide 157 prepared from imidazole acetonitrile 155 protected as its trityl derivative 156, to give trityldidemnimide A 158 and B 159 respectively. Deprotection released the natural products 151 and 152.
Four topsentin class alkaloids have been obtained from the Korean marine sponge Spongosorites genitrix.51 They are all related to the parent alkaloid topsentin 160 being bromodeoxytopsentin 161, isobromodeoxytopsentin 162, which are new. Also present were deoxytopsentin 163 and bromotopsentin 164. A total synthesis of topsentin 160 has been realised by a cross coupling reaction at position 5 using a palladium catalyst and an acylation at position 2 of the imidazole ring.52
Four didemnolines have been synthesised by coupling the appropriate carboline 165/166 with 1-[(benzyloxy)methyl]-4-chloromethyl-5-(thiomethyl)imidazole 167. Removal of the protecting group gave either didemnoline A 169 or B 168 which if followed by periodate oxidation gave the C 170 and D 171 alkaloids.53
A chiral synthesis of the trimethyl ether of imbracatine 172 has confirmed the structure as being a combination of a benzyltetrahydroisoquinoline moiety with an imidazole ring system.54 The connection between the two involved sulfur and this implied that 3-methyl-L-histidine 173 was biogenetically involved. This synthesis, however, involved introducing the sulfur into the isoquinoline moiety with subsequent addition of ‘imidazole’.
Seven new imidazole alkaloids have been identified in the seeds of Lepidium satisvum.55 Two are monomeric imidazole alkaloids namely semilepidinoside A 174 and semilepidinoside B 175 while the others are the bisimidazoles lepidine B 177, lepidine C 178 and lepidine D 179, while lepidine E is 180 and lepidine F is 181. The parent alkaloid lepidine A 176 was also present.
The extract of the Caribbean sponge Agelas conifera, when subjected to a bioassay guided fractionation, led to the identification of three active alkaloids, all belonging to the septrin class. Debromoseptrin 182 is new and was accompanied by monobromoseptrin 183 and by septrin 184 itself.56 All three alkaloids showed activity against Mycobacterium tuberulosis.
Axinellamines A to D are four novel imidazo-azolo-imidazole alkaloids found in an Australian marine sponge of the Axinella family.57 Bioassay active fractions all produced similar consistent ion peaks at 846 and 860 in their electrospray mass spectra. Large scale fractionation led to four active alkaloids, axinellamine A 185, axinellamine B 186, axinellamine C 187 and axinellamine D 188.
Epothilones e.g.189 are of particular interest pharmacologically since they bind to tubulin and thereby they exert an influence, i.e. inhibition, on cell division. A comprehensive paper![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 58 on the synthesis of 26-hydroxyepothilone B 190 first utilises the synthesis of epothilone derivative 191 which upon epoxidation produces the natural product 190. A ‘biological’ synthesis of epothilones has been described whereby the use of enzyme catalysed reactions are employed to custom synthesise chiral intermediates. The stereochemistry for epothilones is mostly associated with the lactone macrolide moiety 189/199. A chiral precursor 193 was synthesised by a retro-aldol–aldol reaction catalysed by an antibody enzyme catalyst 38C2. Racemic 192 was converted into a single diastereoisomer 193 in 60% yield. In a similar procedure using 38C2 aldehyde 194 was chirally converted with acetone, giving 195 with a 75% ee and thus 196 was made. Conventional chemistry produced 197 which was condensed with 196 to give 198. Ring closure with Grubb’s catalyst and deprotection gave epothilone C 199.59
58 on the synthesis of 26-hydroxyepothilone B 190 first utilises the synthesis of epothilone derivative 191 which upon epoxidation produces the natural product 190. A ‘biological’ synthesis of epothilones has been described whereby the use of enzyme catalysed reactions are employed to custom synthesise chiral intermediates. The stereochemistry for epothilones is mostly associated with the lactone macrolide moiety 189/199. A chiral precursor 193 was synthesised by a retro-aldol–aldol reaction catalysed by an antibody enzyme catalyst 38C2. Racemic 192 was converted into a single diastereoisomer 193 in 60% yield. In a similar procedure using 38C2 aldehyde 194 was chirally converted with acetone, giving 195 with a 75% ee and thus 196 was made. Conventional chemistry produced 197 which was condensed with 196 to give 198. Ring closure with Grubb’s catalyst and deprotection gave epothilone C 199.59
In an investigation into the biosynthesis of the marine cyanobacterial metabolite bardamide 200 it has been established that the origin of its trichloromethyl group involves the chlorination of an unactivated pro-S-methyl group of leucine (or a leucine derived intermediate). The authors suggest![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 60 that a radical type reaction may be involved although enzymatic haloperoxidases have hitherto been thought to only utilise cationic halogen.
60 that a radical type reaction may be involved although enzymatic haloperoxidases have hitherto been thought to only utilise cationic halogen.
A new synthesis of (+)-curacin A 201 adopts a novel approach utilising a selective and facile thioacylation of amino-alcohol 202 with the benzotriazole derived thioamide 203. The resulting thioamide 204 was cyclised to the natural product 201 with the Burgess reagent.61 Lyngbyapeptin A 205 contains a 3-methoxybut-2-enoyl moiety which is rare in natural products. The source of this thiazole-containing tetrapeptide is the cyanobacterium Lyngbya bouillionii.62 Dolastin 10 206 is currently undergoing clinical cancer trials at the phase 1 and 11 stage.63
Full details of the total synthesis of (−)-pateamine A 207 and related compounds have appeared.64 The procedure involved synthesis of the thiazole β-lactam synthon 208, addition of the en-yne-side chain 209 which cyclised to 210 which upon reduction to the Z-ene and addition of the side chain by Stille coupling gave (−)-TCBoc-pateamine A 211.
Zelkovamycin 212 is a new antibiotic isolated from the fermentation broth of Streptomyces species K96-0670.65 Four new cyclic peptides have been obtained from the ascidian Lissoclinum patella.66 They are patellamide G 213, ulithiacyclamide E 214, ulithiacyclamide F 215 and ulithiacyclamide G 216. A shake culture of Cystobacter fuscus produces a series of antifungal agents – one of the active ingredients is the novel compound YS1-40-2 217.67
Cystothiazole A 218 and cystothiazole B 219 are new bithiazole-alkaloids isolated from the myxobacterium Cystobacter fuscus.68 A second publication![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 69 cites cystothiazoles C, D, E and F which are closely related being 220, 221, 222 and 223, respectively.
69 cites cystothiazoles C, D, E and F which are closely related being 220, 221, 222 and 223, respectively.
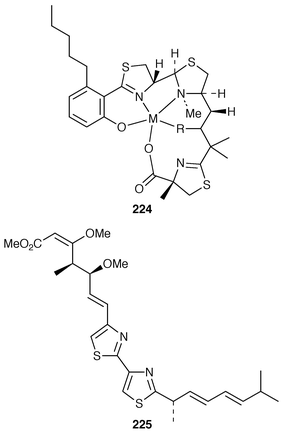
Micococidins A, B and C are antimycoplasma agents isolated from the culture filtrate of Pseudomonas species No. 57-250.70 These novel compounds are metal containing, differing only as to whether they contain Zn2+, Cu2+ or Fe3+224. KR025 225 is another cytotoxic compound isolated from the myxobacterium Micrococcus fulvus. It was isolated from an acetone extract of the cell mass after cultivation.71
Thiazolines ulbactin A 226, ulbactin B 227 and ulbactin C 228 are produced by fungi of the Vitrio and Alteromonas genera. They have found a use as UV-absorbers in cosmetics and as such have been patented.72 The new alkaloids ulbactin D 229 and ulbactin E 230, which are manufactured from an Alteromonas species D-12 (FERMP-15733) have also been patented as UV absorbers for use in cosmetics.73
The total synthesis of micrococcin P 231 was constructed by assembling the four indicated fragments A, B, C and D.74
Designated MJ347-81F4A 232 and MJ347-81F4B 233 these compounds are novel antibiotics obtained from the Amycolatopsis genus.75 They were found in the mycelial cake of species MJ347-81F4.
4 Peptide alkaloids
A review on the classification, isolation, identification and properties of peptide alkaloids has been published in a somewhat obscure journal i.e. the Himalayan Chemical Pharmaceutical Bulletin.76A novel aldose reductase inhibitor 234 has been obtained from the alkalophilic Corynebacterium species YUA25.77 It had no antibacterial activity. Dihydrotubastrine 235 and 4-deoxy-7,8-dihydrotubastrine 236 were isolated from the Indo-Pacific marine sponge Petrosia cf. Contignata.78
Mortivinacins A 237, B 238 and C 239 are metabolites produced by the fungus Mortierella vinacea![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 79 which in turn was isolated from a parasitic soil micro-organism Aspergillus sclerotia. Also isolated was methyl 2,4-dihydroxy-3,5,6-trimethylbenzoate. A polar extract of the leaves of Justicia ghiesbreghtiana has been found
79 which in turn was isolated from a parasitic soil micro-organism Aspergillus sclerotia. Also isolated was methyl 2,4-dihydroxy-3,5,6-trimethylbenzoate. A polar extract of the leaves of Justicia ghiesbreghtiana has been found![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 80 to contain an α-malamic acid derivative 240. The moss Fontinalis squamosa contains a new propionic acid derivative
80 to contain an α-malamic acid derivative 240. The moss Fontinalis squamosa contains a new propionic acid derivative![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 81 namely fontinalin 241.
81 namely fontinalin 241.
A leaf opening substance has been isolated from the myctinastic plant Albizzia julibrissun Durazz. It, cis-p-coumaroylagmatine 242, is specific to this plant causing its leaves to open in darkness![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 82 at a concentration as low as 5 × 10−6 M. The leaf closing process is influenced by another metabolite as yet unidentified (see last year’s Nat. Prod. Rep.).
82 at a concentration as low as 5 × 10−6 M. The leaf closing process is influenced by another metabolite as yet unidentified (see last year’s Nat. Prod. Rep.).
A new amide has been obtained from the bark of Zanthoxylum armatum,83 called armatamide, it is 243. The amide magnolamide 244 is new and has been extracted from the leaves of Magnolia coco.84 Through an insecticidal bioassay procedure five active isobutylamides have been obtained from the ariel parts of Dinosperma erythrococca. Identified as erythrococcamides A 245, B 246 and C 247, which represent a new class of bioactive compounds,85 they were accompanied by (2E,4E)-N-(2-hydroxy-2-methylpropyl-6-phenyl-2,4-hexa-2,4-dienamide 248, and (2E,4E)-N-(2-methylpropyl)-6-phenylhexa-2,4-dienamide 249.
Two novel macrolide containing antitumour metabolites,86 oximidine I 250 and oximidine II 251 have been isolated from Pseudomonas species Q52002. Apicularen A 252 and apicularen B 253 are cytostatic macrolides obtained from a Chomdsomyces robustus species. An extract of this myxobacterium, strain Cma13, after culture, showed no antimicrobial activity but had a high cytotoxity to cultivated and animal cells. A bioassay directed fractionation led to the isolation of these two metabolites; B being much less active than A 0.1–3 μg ml−1vs 0.2–1.2 μg ml−1 respectively.87
The absolute configuration of UK-2A 254, an antibiotic with antifungal activity, has been determined by elucidating the stereochemistry of the butanolide derived from portion A of the macrolide ring and that of the serine portion B.88 Antibiotic NA30851A 255 is elaborated by Streptomyces species NA30851A. It has potential as a bacterioside and insecticide.89 Four metabolites isolated from two species of Australian sponge of the Amphimedon genus, directed by a bioassay fractionation, have nematodal activity.90 Called amphilactams, A is 256, B 257 while C is 258 and D is 259.
Using a convergent approach the synthesis of dolastin 15 260, a linear peptide, has been achieved by involving a solid phase procedure for coupling an N-methylamino acid 261 with pyrrolidine 262. This coupling, to an α,α-dialkylamine, is normally difficult but the use of 1-hydroxy-7-azabenzotriazole (HOAt) together with 2-chloro-1,3-dimethyl-2-imidazolinium hexafluorophosphate (CIP) gave high yields without racemisation.91
Symplostatin 1 is a dolastatin 10 analogue obtained from the marine cyanobacterium Symploca hydnoides.92 This new metabolite 263 is also solid tumour selective in its antineoplastic activity and its isolation from a bacterium suggests that other dolastatin type metabolites found in the sea hare Dolabella may be of dietary origin. A metabolite PF1191 264, obtained by culturing fungus Eupenicillium shearii species PF1191, has the ability to inhibit damage to nerve cells.93 Koromicin is an antibiotic that is specific for Gram-negative marine bacteria through being able to inhibit strongly the respiratory Na+-translocating NADH. This active antibiotic, 265, was obtained from the micro-organism Pseudoalteromonas F.420.94
Jelly meat formation by Sporoza can be an important factor in the manufacture of meat and fish pastes. Its inhibition can be achieved by the addition of a metabolite, compound 460B 266, produced by strain ATCC20386 of the fungus Aspergillus oryzae. Its ability to prevent thiol protease activity results in any food product containing this metabolite (0.005%) exhibiting high jelly strength and elasticity.95
Chlorosis inducing metabolites have been produced by the fungus Pseudomonas syringae.96 They are related to the phytotoxin coronatine 267 being N-coronafacoyl-L-serine 268 and N-coronafacoyl-L-threonine 269.
α-MAPl is a protease inhibitor which is accompanied by its β- and γ-analogues in a mixture called microbial alkaline proteinase inhibitor which is produced by Streptomyces nigrescens. The synthesis of α-MAPl 270 has been accomplished![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 97 using a solid support whereby 271 combined with protected arginine, followed by the addition of valine, suitably protected, and then phenylalanine diol 272 gave the resin peptide 273. Removal from the resin support gave the peptide 274 which when followed by reductive removal of the nitro group gave diol 275, periodate cleavage subsequently gave α-MAPl 270.
97 using a solid support whereby 271 combined with protected arginine, followed by the addition of valine, suitably protected, and then phenylalanine diol 272 gave the resin peptide 273. Removal from the resin support gave the peptide 274 which when followed by reductive removal of the nitro group gave diol 275, periodate cleavage subsequently gave α-MAPl 270.
A revised structure for flavolipin 276 has been proposed following a synthetic investigation.98 In all, twelve stereoisomers, were synthesised before its structure was confirmed. This confirmation makes flavolipin identical to two other metabolites, namely WB-3559 and topostin D 654.
Four new microginins have been isolated from species of the cyanobacterium Microcystis aeruginosa.99 All are linear peptides thus microginin 299C 277 and microginin 299D 278 came from species NIES-299 while microginin 99A 279 and microginin 99B 280 came from species NIES-99. Fontaineine 281 is a new alkaloid obtained from the leaves of Fontainea pancheri.100 Aranaclor A 282 and aranaclor B 283 are two new metabolites found in the fermentation fluid of Pseudoarachniotus roseus. Both inhibit a range of bacterial and fungal strains.101
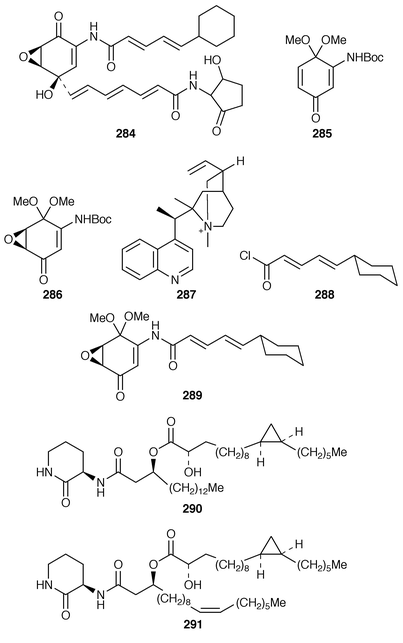
A synthesis of (−)-alisamycin 284 requires, as a key step, introduction of the oxirane ring stereospecifically into a quinone precursor. This has been elegantly achieved employing a chiral phase-transfer reagent.102 Thus 285 was epoxidised stereospecifically to 286 in 39% yield using catalyst 287. Deprotection allowed condensation with acid chloride 288 to give 289 and thence (−)-alisamycin 284. A revision of the structures of cepaciamide A 290 and cepaciamide B 291 has been suggested![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 103 and confirmed by synthesis.104
103 and confirmed by synthesis.104
Aeruginosin 103-A 292 is a thrombin inhibitor obtained from the cyanobacterium Microcystis viridis.105 A solid state fermentation of the vegative mycelia of strain YL-03706F from Candida tropicalis has produced![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 106 a novel lipopeptide antibiotic designated YM-170320 293. Sinulamide 294 is an H,K-ATPase inhibitor. Isolated from a soft coral of the Sinularia group, its structure was assigned on the basis of spectroscopic data followed by confirmation through total synthesis.107 The fungus Streptomyces griseus species RK-1009 produces not only the antibiotic factumycin 295 but its stereoisomer A73A 296 as well as a new derivative, the 3-O-methyl-analogue 297.108
106 a novel lipopeptide antibiotic designated YM-170320 293. Sinulamide 294 is an H,K-ATPase inhibitor. Isolated from a soft coral of the Sinularia group, its structure was assigned on the basis of spectroscopic data followed by confirmation through total synthesis.107 The fungus Streptomyces griseus species RK-1009 produces not only the antibiotic factumycin 295 but its stereoisomer A73A 296 as well as a new derivative, the 3-O-methyl-analogue 297.108
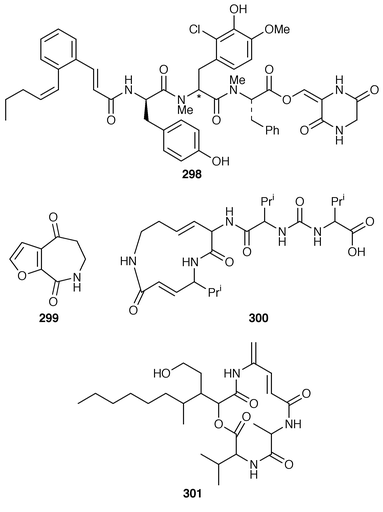
The synthesis of the farnesyl transferase inhibitor pepticunnamin E 298, previously produced by a Streptomyces species, has created the metabolite itself and its diastereomer, at the centre indicated (*). Chirality is decisive in the inhibitor properties of these two isomers, the natural product being six times more active than its epi-isomer.109 The furanolactam 299 is new and has been isolated from the Chinese sponge Phacellia fusca.110 Syringolin is a novel peptide elicitor obtained from Pseudomonas syringae pv syringae. It 300 induces resistance to the non-host pathogen of rice (Oryza sativa), namely the blast fungus Pyricularia oryzae.111 Antibiotic vinylamycin 301 can be obtained by culturing Streptomyces species MI982-63Fl.112
The total synthesis of ‘antillatoxin’ did not afford the natural product so the question arises what is the real structure for this metabolite? Since the integrity of the amino-acid components of this tripeptide 302 are secure, it only remains for the stereocentres at C-4 and C-5 to be responsible for the disparity between the synthetic product and the natural one; more work follows.113 Another attempt at the synthesis of antillatoxin 302 also resulted in its epimer being produced. From these results and a re-examination of the NMR data for the natural product it is suggested that C-4 and C-5 are ‘trans’ related.114 Aburatubolactam C 303 is a novel apoptois-inducing substance produced by a Streptomyces species SCRCA-20 which was obtained from a marine mollusk.115 The group of substances designated BE-43547 are based on the macrocyclic peptide system 304. They all possess antitumour properties and were manufactured from a Streptomyces fungus strain A43547.116 Geodiamolides H and I are two new cyclodepsipeptides isolated from a marine sponge of the Geodia family. Structural determination and absolute stereochemistry were confirmed by X-ray crystallography.117 Thus H was 305 and I 306.
Astin D 307 has been synthesised for the first time.118 Proline methyl ester hydrochloride salt 308 was coupled with α-aminobutyric acid 309 to give, after hydrolysis, amino-acid 310. Condensation with dipeptide 311 gave tetrapeptide 312. Deprotection gave the appropriate amino-acid which upon treatment with TBTU ([2-(1H-benzotriazol-1-yl)]-1,1,3,3-tetramethyluronium tetrafluoroborate) in 2-methylpropan-2-ol followed by DIEA, after 8–15 hours produced the macrolide astin-O-benzyl ether 313. Deprotection resulted in the natural product 307 being formed.
The limiting factor in yields can be the difficulty in ring closing tetra or larger peptides to the macrocycle. In a study on the synthesis of cyclosporine 314 it has been found that the resin-bound heptapeptide precursor could be detached and cyclised in situ.119 It is best to use NMP as solvent, as it gives much shorter reaction times, days not months!
Two new cyclopeptides have been isolated from whole plants of Dianthus superbus.120 Called dianthin A 315 and dianthin B 316 their structures were determined by 2D NMR techniques. Dehydrodidemnin B 317 is the most potent member of this family of macrocyclic depsipeptides. Its synthesis is particularly important since it is the minor component of the group. Two routes have been reported![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 121 which both generated first didemnin A 318 which was then coupled with proline MeCOCO–Pro-OH to give B 317.
121 which both generated first didemnin A 318 which was then coupled with proline MeCOCO–Pro-OH to give B 317.
Two new jaspamide derivatives have been obtained from the marine sponge Jaspis splendans![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 122 called jaspamide B 319 and jaspamide C 320, they both showed cytotoxic activity against human NSCLC-N6 cancer cell lines.
122 called jaspamide B 319 and jaspamide C 320, they both showed cytotoxic activity against human NSCLC-N6 cancer cell lines.
Since the synthesis of (4R,11R)-cyclocinamide A 321 gave rise to a heptapeptide which was not that of the natural product, cinamide A is now thought to be the 4S, 7S, 11S, 14S) stereoisomer.123 Micropeptin T-20 is a novel phosphate containing cyclic depsipeptide 322 isolated from the cyanobacterium Microcystis aeruginosa.124 Cyclopeptide TMC95A 323 is produced by fermentation of the micro-organism designated Apiospora or Arthrinium montagnei. Being a proteasome inhibitor it is claimed to be useful for the treatment and/or prevention of chronic articular rheumatism.125 Streptovaricin C 324 has been obtained from the soil micro-organism Streptomyces species KMl-30.126
Depsipeptide elastase inhibitors YH-47141 325 and YM-4742 326 contain the interesting hydrated vicinal tricarbonyl grouping. Their synthesis required creation of the tricarbonyl system in protected form. To this end a ketoamide 327 was converted into ylide 328 which upon treatment with triethylamine in methylene chloride, followed by addition of protected leucine gave 329, a protected tricarbonyl synthon. Further condensation, ring closure and attachment of the side chain resulted in the synthesis of the protected tricarbonyl analogue of the natural product. Ozonolysis in methylene chloride at −78 °C produced the respective depsipeptide in two minutes.127
The new peptide 330 from Streptomyces nobilis has both antitumouric and antiinflammatory properties.128 Jiperamaishin 331 is an antitumouric antibiotic isolated from Streptomyces species MK393-AF2.129 A series of novel hexadepsipeptides have been isolated from Streptomyces nobilis.130 They are based on structure 332 where R is H or a lower alkyl group.
The total synthesis of luzopeptins A 333, B 334 and C 335, the antitumour antibiotics with antiviral properties as well, has been completed in a convergent manner.131 Thus (3R)-N-methyl-hydroxyvalinol (as its THP ether) 336 was condensed with protected glycylsarcosine 337. Deprotection and oxidation gave a carboxylic acid which was reprotected at nitrogen by the FMOC group, 338. Condensation with protected dipeptide 339 gave 340 which on N-deprotection gave 341 and readjustment of the protecting groups then gave 342. Coupling of 341 and 342 produced the linear decadepsipeptide 343 whence N-deprotection and carboxy deprotection gave amino acid 344 which on treatment with EDCl in ethyl acetate gave the cyclic product 345. Removal of the remaining protecting groups released luzopeptin C 335 which on mono- or di-acetylation gave A 333 and B 334 respectively.
Salinamide A 346 and salinamide B 347 are two depsipeptides possessing antiinflammatory activity. Both were found in a marine based Streptomyces fungus, strain CNB-091, isolated from a jellyfish.132 An unidentified fungus produces a cyclodepsipeptide Sch217048 348 which has neurokinin antagonist activity.133 Cyclic hexapeptides having antibiotic activity have been obtained from the fungus Coleophoma species F-11899. The metabolites, based on 349, are active against Pneumocystic carinii pneumonia human and animal pathogens.134
Dolastin 12 350 and lyngbyastatin l 351 have been isolated from the cyanobacterial assemblages Lyngbya majuscula/Schizothrix calcicola.135 Both metabolites have the same ability to isomerise slowly in solution at the C-15 carbon. Antifungal cyclodepsipeptide cyclolithistide A 352 has been isolated from the marine sponge Theonella swinhoei. It contains three unique amino acids, namely 4-amino-3,5-dihydroxyhexanoic acid, formylleucine and chloroleucine.136
Two new microcystins have been obtained from a hepatotoxic bloom of Oscillatoria agardhii found at Soulseat loch in Scotland.137 Both contain a Z-dehydrobutyrine grouping, the first is [D-Asp3,(Z)-Dhb7]microcystin-Hty R 353 and the second [D-Asp3,(Z)-Dhb7]microcystin-LR 354. The structure for himastatin 355 has been confirmed by synthesis![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 138 but has to be revised stereochemically whereby the relationship between the amide grouping and the C-2 hydrogen is α and not as previously reported β.139
138 but has to be revised stereochemically whereby the relationship between the amide grouping and the C-2 hydrogen is α and not as previously reported β.139
Eurypamide A is a cyclic tripeptide 356 isolated from the Papuan New Guinea sponge Microciona eurypa.140 It is one of four seventeen-membered macrocyclic tripeptides containing an isodityrosine component. The other three, B, C and D were inseparable.
An asymmetric total synthesis of sanjoinine Gl 357 involved first the synthesis of a fluoro-nitrophenyl peptide 358 which upon treatment with TBAF and a 3 Å molecular sieve under dilute conditions (0.01 M) in DMSO cyclised to 359 (the nitro-group activates its neighbour fluorine for ether formation). Interestingly, only one atropoisomer of 358 cyclised. Modification of the cyclic peptide ether resulted in sanjoinine Gl 357 production.141 Another synthesis of sanjoinine Gl 357 has been reported,142 in this case ether formation was accomplished by activating a phenol with a para-cyano-group. Thus 4-cyanophenol 360 easily condensed with amino-alcohol 361 to give 362 in 85% yield. Elaboration giving sanjoinine Gl 357.
Novel secondary metabolite RP66453 363, obtained from Streptomyces strain A9738, is capable of binding to the neurotensin receptor of the guinea pig.143 As such it is suggested that it is a constrained neutrotensin (8-13) analogue.
Five communications describe the total synthesis of the aglycone of vancomycin 364 R![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) H, three by the Nicolaou group
H, three by the Nicolaou group![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 144–146 and two by the Evans group.147,148 The introduction of the sugar unit on to the aglycone of vancomycin 364 has hitherto circumvented the total synthesis of this antibiotic. It is now reported that the pseudoaglycone of vancomycin 365 produced by mild acid treatment of vancomycin 366 can, after protection, be converted back into vancomycin 366 itself. The protecting groups render the antibiotic precursor 365 soluble in non-polar solvents and so amenable to chemical manipulation.149
144–146 and two by the Evans group.147,148 The introduction of the sugar unit on to the aglycone of vancomycin 364 has hitherto circumvented the total synthesis of this antibiotic. It is now reported that the pseudoaglycone of vancomycin 365 produced by mild acid treatment of vancomycin 366 can, after protection, be converted back into vancomycin 366 itself. The protecting groups render the antibiotic precursor 365 soluble in non-polar solvents and so amenable to chemical manipulation.149
5 Miscellaneous alkaloids
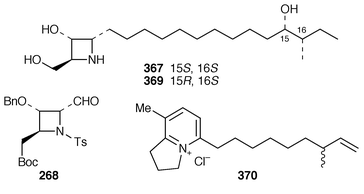
A novel enantioselective synthesis of penaresidin A 367 has been achieved using a highly functionalized azetidine containing all the requisite stereogenic centres 368. This synthon was also used![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 150 to synthesise allo-penaresidin 369. Louludinium chloride 370 is an azabicyclononane alkaloid obtained from the blue–green alga Lyngbya gracilis. This rarely encountered type of metabolite had moderate antitumour activity.151
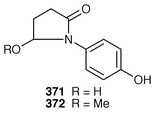
150 to synthesise allo-penaresidin 369. Louludinium chloride 370 is an azabicyclononane alkaloid obtained from the blue–green alga Lyngbya gracilis. This rarely encountered type of metabolite had moderate antitumour activity.151Lapiotin A 371 and lapiotin B 372 are produced by the poisonous mushrooms Chlorophyllum molybidilis and Macrolepiotia neomastoidea.152
The substance which causes the leaf opening of Phyllanthus urinaria is phyllurine 373. It is highly specific for its host at a concentration of 2.5 × 10−5 M. The leaf closing substance for this plant is non-alkaloidal phyllanthurino-lactone 374. The interrelationship between these two substances is being investigated.153
A new dibromotyrosine derivative 375 has been identified in the extract from the sponge Psammaplysilla purpurea.154 Three known bromotyrosine metabolites were also present in the extract.
An efficient total synthesis of (−)-balanol 376, the potent protein kinase inhibitor, has been published.155 It uses a radical cyclisation procedure to generate the hexahydroazepine component 379. Thus oxime 377 cyclised to 378 and 379 in the presence of Bu3SnH, the ratio being 1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ∶
∶![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 3. Using SmI2 in HMPA the ratio was 1
3. Using SmI2 in HMPA the ratio was 1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ∶
∶![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 7, both radical reactions having ≅50% yields. The favoured isomer 379 condensed, after deprotection, with p-(benzyloxy)benzoyl chloride to give racemic 380. Formation of the chiral ester with NBoc-L-alanine 381 enabled the diastereoisomers to be separated by chiral chromatography. An alternative resolution procedure was to take the diastereomeric alcohol 380 and chirally acetylate it with an immobilized lipase, a Pseudomonas species being the most efficient, which in the presence of vinyl acetate produced acetate 382 with 3S,4S leaving the 3R,4R isomer unacetylated (ee 97%; 42% yield). Chromatography resulted in a easy separation of the two compounds. The benzophenone moiety 383 was synthesised by a biomimetic designed procedure starting with natural chrysophanic acid 384. Methylation gave 385 which was followed by reductive methylation that afforded 386 which upon oxidative ring opening, utilising singlet oxygen, gave 387. Conversion of 387 into protected chrysophanic acid 388 was achieved by standard procedures whence condensation with 379 (3R,4R) and removal of the protecting groups produced (−)-balanol 376.
7, both radical reactions having ≅50% yields. The favoured isomer 379 condensed, after deprotection, with p-(benzyloxy)benzoyl chloride to give racemic 380. Formation of the chiral ester with NBoc-L-alanine 381 enabled the diastereoisomers to be separated by chiral chromatography. An alternative resolution procedure was to take the diastereomeric alcohol 380 and chirally acetylate it with an immobilized lipase, a Pseudomonas species being the most efficient, which in the presence of vinyl acetate produced acetate 382 with 3S,4S leaving the 3R,4R isomer unacetylated (ee 97%; 42% yield). Chromatography resulted in a easy separation of the two compounds. The benzophenone moiety 383 was synthesised by a biomimetic designed procedure starting with natural chrysophanic acid 384. Methylation gave 385 which was followed by reductive methylation that afforded 386 which upon oxidative ring opening, utilising singlet oxygen, gave 387. Conversion of 387 into protected chrysophanic acid 388 was achieved by standard procedures whence condensation with 379 (3R,4R) and removal of the protecting groups produced (−)-balanol 376.
Three solanapyrones, designated E, F and G, are antialgal metabolites produced by a marine fungus found on the surface of the green alga Halimeda monile.156 The fungus was of the filamentous type but was unidentified; the metabolite structures are 389, 390 and 391 respectively. A new antibiotic, called viridomycin F 392, has been isolated from the culture fluid of Streptomyces species K96-0188.157
The synthesis of mymicarin 217 393, a pyrrolo[2,1,5-cd]indolizine produced in the poison gland of Myrmicara ants, has been achieved using biogenetic principles.158 Starting with 6-methylpyridine-2-carbaldehyde, intermediate 394 was produced which was converted into 395. Deprotection gave diketone 396 which spontaneously cyclised slowly to mymicarin 217. Indolizidine 397 was an intermediate in the cyclisation process.
Cladobotryal has a novel alkaloid structure 398. This antifungal metabolite was found through a screening programme applied to the culture fluid from the mycoparasitic fungus Cladobotryum rarium.159 Dehydroradiosumin 399, obtained from the fresh water cyanobacterium Anabaena cylindrica, is a trypsin inhibitor.160 A leaf opening substance from Mimosa pudica called mimopudine, 400, appears to have its effect on the slow leaf closing movement – the nyctinastic effect. The other movement is much faster – touch induced not light induced. On this basis it is thought that different substances influence these leaf movements.161 Tuberostemoenone 401 is a new alkaloid obtained from the roots of Stemona tuberosa found in the North part of China.162 Its structure was elucidated by 2D-NMR and computer modelling confirmed the NMR interpretation. Epostatin is an inhibitor of dipeptidyl peptidase. It, 402, was isolated from a species (MJ995-OF5) of Streptomyces.163
Equisetin 403 and phomasetin 404 are two fungal metabolites with the ability to inhibit the HIV-1 integrase process. These two alkaloids, although similar, have different stereochemistry at some of their chiral centres.164 Two heptaene antibiotics designated 3874H1 405 and 3874H3 406 have been isolated from the fungus Streptomyces sp. HAG OO3874.165
The total synthesis of cyclic marine alkaloids halidamine A 407 and halidamine B 408 have been brought about by reacting the deactivated pyridine mesylate 409 with pyridine derivative 410 yielding 411 which was deprotected to give 412, mesylated to yield 413, deoxygenated to afford 414 and cyclised to 415. This compound 415 was converted into 407 by sodium borohydride reduction; 408 was synthesised by the same methodology.166
The macrocyclic spermine alkaloids verbacine 416 and verballocine 417 were isolated from Verbascum pseudonobile and their N-acylated analogues 418 and 419 from Verbascum phoeniceum. All four have now been found in this latter plant. Treatment of either alkaloid 416 or 417 with formaldehyde gave the N,N′-methylene bridged product 420 and 421 respectively. Both have now been isolated as natural products.167 Comprehensive chiroptical analyses of these and other spermidines are also reported in this publication. Kukoamine A 422 is a spermine alkaloid found in the dried root bark of Lycium chinense. A new synthetic procedure allows all four regioisomers to be prepared. The method employed a solid support (2-chlorotrityl resin).168
Metabolite BE-55051 is produced by cultivation of Streptomyces species A55051. It, 423, shows good antitumour properties.169 Granulatimide 424 and isogranulatimide 425 are aromatic alkaloids isolated from the Brazilian ascidian Didemnum granulatum.170 They are essentially cyclised didemnides – alkaloids which co-occur in this ascidian. A cyclised didemnimide alkaloid has also been found in the Caribbean ascidian Didemnum conchyliatum. It is identical to isogranulatimide 425. Its precursor is probably didemnimide A 426 which was shown to cyclise to 425 through UV irradiation in a quartz vessel.171
A new synthesis of staurosporinone 427, taking six steps, has been reported.172 This natural product is regarded as the aglycone for staurosporine 428 and related alkaloids. Noelaquinone 429 is a triazine quinone found in an Indonesian sponge belonging to the Xestospongia family.173 Halitulin 430 is a new cytotoxic alkaloid found in the marine sponge Haliclona tulearensis.174
It is possible to obtain a new 2,5-diketopiperizine derivative using a new isolate from the blackleg fungus Phoma lingam. It, 431, is different to previous diketopiperizines isolated from blackleg fungi.175
A synthesis of the bioactive alkaloid (±)-aglaiastin 432 has been realised utilising an already synthesised ABC tricyclic synthon. Thus 433 was condensed with 4-aminobutyraldehyde diethylacetal to give 434 which was oxidised to ketone 435 using SO3–pyridine at rt. Cyclisation of 435 to give (±)-aglaiastin 432 occurred by treating 435 successively with 1 M HCl, formic acid and ammonium formate in methanol.176
The crambescidins constitute a group of antiviral and cytotoxic metabolites produced by the sponge Crambe crambe. A patent![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 177 has been taken out on a number of these metabolites whose structure is based on 436. Roquefortine D 437 is the minor alkaloid produced along with roquefortine C 438 by the fungus Penicillum rogueforti. It can be converted into 437 by reduction with zinc in acetic acid. A synthesis of this minor alkaloid
177 has been taken out on a number of these metabolites whose structure is based on 436. Roquefortine D 437 is the minor alkaloid produced along with roquefortine C 438 by the fungus Penicillum rogueforti. It can be converted into 437 by reduction with zinc in acetic acid. A synthesis of this minor alkaloid![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 178 starts with L-tryptophan methyl ester 439. Conversion by established procedures gave 440 which then gave with the histidine derivative 441 the peptide 442. Boc deprotection enabled cyclisation to take place at rt in methanol–ammonia over 3 days giving 443 which could lose its o-nitrobenzyl (ONB) protecting group, by irradiation, to give roquefortine D 437.
178 starts with L-tryptophan methyl ester 439. Conversion by established procedures gave 440 which then gave with the histidine derivative 441 the peptide 442. Boc deprotection enabled cyclisation to take place at rt in methanol–ammonia over 3 days giving 443 which could lose its o-nitrobenzyl (ONB) protecting group, by irradiation, to give roquefortine D 437.
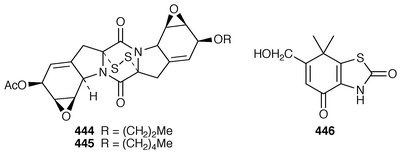
Ambewelamides A 444 and ambewelamide B 445 are two new antineoplastic metabolites obtained from a Sri Lankan lichen of the Usuea group.179 Thiazinedione 446 has been isolated from an aqueous acetone extract of the burweed fruit. This fruit, used in traditional Chinese medicine to treat rheumatism and skin pruritis, comes from the tree Xanthium strumarium.180
The antimalarial alkaloids febrifugine and isofebrifugine have been synthesised starting with simple chiral compounds and as a result their structures have been revised.181 The first synthesis used aldehyde 447 to make 449 which led to 450, which was previously thought to be febrifugine but it had the opposite optical rotation. On the other hand using 448, the S-isomer, the synthetic product via451, was identical in all respects with naturally occurring febrifugine – hence its structure is 452. Similarly isofebrifugine was synthesised from 447 and is 453.
A marine bacterium isolated from tissues removed from a Palythoa species, when grown artificially, has been able to produce two plant growth promoters.182 They are the diketopiperazines 454 and 455. Antitumour NSCL-86F037 456 can be obtained by culturing Aspergillus ustus.183 It is particularly effective in preventing cell cycle and growth in tumour cells. Okaramine G 457 has been isolated from the culturing of Penicillium simplicissimum ATCC90288. It is reported to have an insecticidal activity against silkworms.184 A synthesis of what was thought to be lepadiformine 458 did not produce the natural product.185 Clavulazine 459 is a new marine pyrazine congener isolated from the soft coral Clavularia viridis.186 An acyl CoA synthetase inhibitor has been obtained from the culture broth from a Bacillus, species B-6. It is phenazine-1-carboxylic acid 460.187 APHE’s are supposed to be pyrazoisoquinolines produced by the actinomycete Streptoverticillium grisecarneum. Four of them are known all showing variation as to their alkyl substituent 461. A new investigation into the synthesis of these alkaloids reports![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 188 that they are better represented by the ring structure 462.
188 that they are better represented by the ring structure 462.
A novel process for preparing antitumour active metabolites based on compound FR900482 463 has been achieved by incubating the alcoholic metabolite(s) 464 with an oxidase-producing Fusarium culture filtrate.189 Oxidation to 465 occurs specifically on a variety of derivatives where R1 is H, alkanoyl, alkyl.
A submerged fermentation culture of Streptomyces armeniacus, strain ATCC 15676, produces an antimicrobial metabolite![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 190 called streptopyrrole, it has structure 466.
190 called streptopyrrole, it has structure 466.
Euthyroideones are novel brominated quinone methides isolated from the Bryozoan sponge Honophymia conferta.191 Three alkaloids have been characterised: A is 467, B is 468 and C is 469. Sodium aristolochate 470 has been obtained from the leaves of Aristolochia foveolata. The two other aristolochic acid sodium salts that were also present in the extract![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 192 were 471 and 472. The fresh leaves of Aristolochia kaempferi
192 were 471 and 472. The fresh leaves of Aristolochia kaempferi![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 193 contain aristolukine-A 473 and aristolukine-B 474.
193 contain aristolukine-A 473 and aristolukine-B 474.
A micro-organism which is described as a biologically pure culture of Streptomyces rimosus X10/79/978 can produce a series of metabolites containing a pyrrole ring fused to a benzoxazine. Thus the basic product 475 has halogen, hydroxy, or alkoxy groups located on the periphery of the molecule.194
The total synthesis of phomazarin 476 has been concisely achieved by employing a heterocyclic azadiene inverse electron demand Diels–Alder reaction![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 195 to create the penta substituted pyridine synthon 478. Condensation of 1,1,2-trimethoxyethylene 477 with triazine 478 occurred smoothly to give 479, this Diels–Alder reaction occurring at temperatures as low as 25 °C – albeit slowly. Hydrolysis, anhydride formation and esterification produced 480 which could be condensed with 481 to give benzophenone 482 which on treatment with sodium borohydride in THF was converted into lactone 483 whence hydrogenolysis gave pyridylphenylmethane 484. TFAA treatment on 484 caused ring closure to give 485 whence oxidation with ozone created benzoquinone 486i.e. 3,4-O-dimethylphomazarin methyl ester. Demethylation with AlCl3 or BCl3 gave monodemethylation 487 which was completely demethylated with toluene-p-sulfonic acid in trifluoromethanol at 100 °C. Hydrolysis of the methyl ester 488 with LiOH completed the synthesis to phomazarin 476 (Scheme 2).
195 to create the penta substituted pyridine synthon 478. Condensation of 1,1,2-trimethoxyethylene 477 with triazine 478 occurred smoothly to give 479, this Diels–Alder reaction occurring at temperatures as low as 25 °C – albeit slowly. Hydrolysis, anhydride formation and esterification produced 480 which could be condensed with 481 to give benzophenone 482 which on treatment with sodium borohydride in THF was converted into lactone 483 whence hydrogenolysis gave pyridylphenylmethane 484. TFAA treatment on 484 caused ring closure to give 485 whence oxidation with ozone created benzoquinone 486i.e. 3,4-O-dimethylphomazarin methyl ester. Demethylation with AlCl3 or BCl3 gave monodemethylation 487 which was completely demethylated with toluene-p-sulfonic acid in trifluoromethanol at 100 °C. Hydrolysis of the methyl ester 488 with LiOH completed the synthesis to phomazarin 476 (Scheme 2).
 | ||
Scheme 2 Reagents and conditions: (i)Dioxane, 100 °C, 30 min then Et3N, 1 h; (ii) LiOH, THF–CH3OH–H2O (3![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ∶ ∶![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 2 2![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ∶ ∶![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 1), 90 °C, 36 h; (iii) TFAA, 25 °C, 2 h; (iv) CH2N2, THF, 0 °C, 5 min; (v) THF, −78 °C, 5 min; (vi) NaBH4, MeOH, −10 °C, 40 min; (vii) H2, Pd(OH)2, EtOH, 25 °C, 2 h; (viii) TFAA, 50 °C, 72 h; (ix) O2, MeOH, 30 °C, 72 h; (x) BCl3, CH2Cl2, −78 °C to 50 °C, 3 h; (xi) TsOH, NaBr, CF3CH2OH, 100 °C, 36 h; (xii) LiOH, THF–H2O (3 1), 90 °C, 36 h; (iii) TFAA, 25 °C, 2 h; (iv) CH2N2, THF, 0 °C, 5 min; (v) THF, −78 °C, 5 min; (vi) NaBH4, MeOH, −10 °C, 40 min; (vii) H2, Pd(OH)2, EtOH, 25 °C, 2 h; (viii) TFAA, 50 °C, 72 h; (ix) O2, MeOH, 30 °C, 72 h; (x) BCl3, CH2Cl2, −78 °C to 50 °C, 3 h; (xi) TsOH, NaBr, CF3CH2OH, 100 °C, 36 h; (xii) LiOH, THF–H2O (3![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ∶ ∶![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 1), 30 °C, 24 h. 1), 30 °C, 24 h.
| ||
A new approach has been carried out to effect a total synthesis of four makaluvamine alkaloids.196 Key pyrrolo-quinoline intermediates 491 and 492 were prepared by aryne-mediated cyclisations of 4-chloro-6-methoxy derivatives 489 and 490. Oxidation with O2–salcomine produced quinone imine 493 which on treatment with ammonium chloride in methanol gave makaluvamine A 495. Tyramine produced makaluvamine K 496 and with intermediate 494 two similar methoxy replacements, after deprotection, produced makaluvamine I 497 and makaluvamine D 498. In a direct, thirteen step procedure, the synthesis of makaluvamine C 499 has been reported.197 4-Anisidine was the starting material and the overall yield was 13%. The synthesis of makaluvamine F 500 has been brought about by the use of hypervalent iodine in a key step for the conversion of 501 into 502. The reagent was a combination of iodosobenzene and trimethylsilylazide i.e. PhI![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O-Me3SiN3.198 Thereafter reaction with core 494 led to makaluvamine F 500.
O-Me3SiN3.198 Thereafter reaction with core 494 led to makaluvamine F 500.
Veiutamine 503 is found in the marine sponge Zyzzya fuliginosa. It can be synthesised![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 199 by a direct lithiation at position 6 of 504 thus allowing attachment of the appropriate side chain thus leading eventually to alkaloid 503.
199 by a direct lithiation at position 6 of 504 thus allowing attachment of the appropriate side chain thus leading eventually to alkaloid 503.
Batzelladine alkaloid syntheses primarily require the creation of the tricyclic quanidine core 505. This has been achieved![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 200 stereospecifically using a Biginelli condensation to transform 506 to a mixture of syn- and anti-isomers at centres 2a and 8a. Thus bicyclic hexahydropyrrolopyrimidium chloride 506 condensed with methyl acetoacetate 507 to give a mixture of syn- and anti-stereomers 505 and 509. Catalytic reduction has, as yet, not been stereospecific and 505n = 8 can be obtained pure only after chromatographic separation of the mixture. However, if the Biginelli precursor 506 is condensed with 507 in the presence of one equivalent of morpholinium acetate the tricyclic methyl ester is mainly 508. Catalytic reduction using Pd/C in methanol converts 508 into 505n = 8 in high yield. Attachment of the appropriate ester side chain gave batzelladine D 509. Other batzelladines were also synthesised by this procedure e.g. batzelladine F 510.
200 stereospecifically using a Biginelli condensation to transform 506 to a mixture of syn- and anti-isomers at centres 2a and 8a. Thus bicyclic hexahydropyrrolopyrimidium chloride 506 condensed with methyl acetoacetate 507 to give a mixture of syn- and anti-stereomers 505 and 509. Catalytic reduction has, as yet, not been stereospecific and 505n = 8 can be obtained pure only after chromatographic separation of the mixture. However, if the Biginelli precursor 506 is condensed with 507 in the presence of one equivalent of morpholinium acetate the tricyclic methyl ester is mainly 508. Catalytic reduction using Pd/C in methanol converts 508 into 505n = 8 in high yield. Attachment of the appropriate ester side chain gave batzelladine D 509. Other batzelladines were also synthesised by this procedure e.g. batzelladine F 510.
Aaptosamine 511 is a new 5,8-diazabenzo[cd![[hair space]](https://www.rsc.org/images/entities/i_char_200a.gif) ]azulene alkaloid isolated from the Caribbean marine sponge Aaptos aaptos.201 Aaptamines, the marine alkaloids usually found in the marine sponge Aaptos aaptos, have had their syntheses reviewed.202
]azulene alkaloid isolated from the Caribbean marine sponge Aaptos aaptos.201 Aaptamines, the marine alkaloids usually found in the marine sponge Aaptos aaptos, have had their syntheses reviewed.202
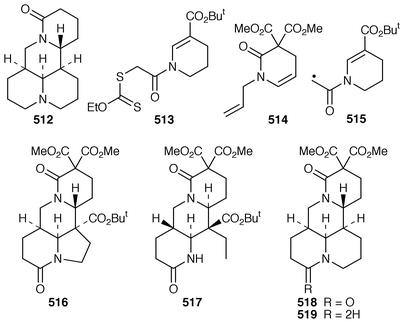
A concise synthesis of (±)-matrine 512 has been realised using a convergent radical cascade reaction.203 After several trial experiments xanthate 513 and dihydroquinolone 514 were chosen as precursors which, when mixed and treated with peroxide, decomposed the xanthate to give radical 515, which with 514 produced two products 516 and 517. Separating chromatographically 516 from 517 allowed it to be decarboxylated by the Barton method with the hydrogen being introduced with the α-configuration 518. Reduction of this lactam 518 delivered 519 which upon hydrolysis and decarboxylation afforded (±)-matrine 512.
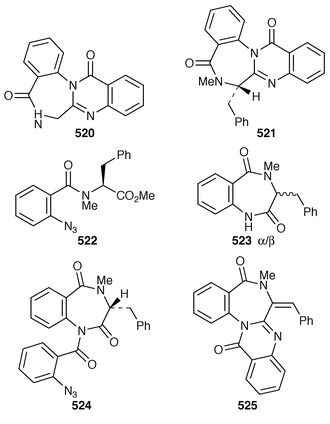
Sclerotigenin 520 is a new benzodiazepine isolated from the sclerotia of Penicilliumsclerotigennin.204 It possesses antiinsectan properties as does griseofulvin which was also found in this sclerotia.
A first time synthesis of (−)-benzomalvin A 521 results from an intramolecular aza-Wittig reaction![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 205 of 522 giving 523 as an α and β mixture which could be isomerized completely to the α-isomer, which was then N-coupled to 2-azidobenzoyl chloride to give 524. This could be cyclised to benzomalvin A 521 when treated with triphenylphosphine. Benzomalvin B 525 was also prepared by first bromination and then dehydrobromination of benzomalvin A.
205 of 522 giving 523 as an α and β mixture which could be isomerized completely to the α-isomer, which was then N-coupled to 2-azidobenzoyl chloride to give 524. This could be cyclised to benzomalvin A 521 when treated with triphenylphosphine. Benzomalvin B 525 was also prepared by first bromination and then dehydrobromination of benzomalvin A.
Two new purple coloured alkaloids called iheyamine A 526 and iheyamine B 527 have been found in a purple coloured ascidian belonging to the Polycitorella species.206 Both compounds showed moderate cytotoxic properties.
Styelsamines A, B, C and D are new tetracyclic pyridoacridine alkaloids isolated from the Indonesian ascidian Eusynstyela latericius.207 Their structures are 528, 529, 530 and 531, respectively.
A biomimetic approach to discorhabdin alkaloids has been suggested because of the relative ease which indoloquinone 532 (R are protecting groups) reacts in TFA with a tyramine to give a makaluvamine e.g.533. Its ability to produce readily the spiro-alkaloidal system e.g.534 lends itself to a viable biomimetic concept,208 thus suggesting that makaluvamine F 500 could be a precursor for discorhabdin D 535. Discorhabdin P 536 is another enzyme inhibitor isolated from a deep water Caribbean sponge of the genus Batzella.209 A new discorhabdin, 537, designated Q has been isolated from two sponge families Latrunculia purpurea and several members of the Zyzzya group namely Z. massalis, Z. fuliginosa and other Z. species.210 All contain this metabolite which is in fact 16,17-dehydrodiscorhabdin B.
The synthesis of meridine 538 and cystodamine 539 can be achieved by a hetero-Diels–Alder reaction. Basically 540 and 541 react in chloroform slowly to give 542 which can then be manipulated to give the natural products.211
A synthesis of pantherinine 543 occurs through a cross-coupling reaction between quinoline 544 and the boronic acid 545 whereby a Suzuki reaction occurred, catalysed by tetrakis(triphenylphosphine)palladium, to make 546; and thence to the pantherinine 543 by conventional procedures.212
To synthesise the cytotoxic alkaloid luotonin A 547, pyridolactam 548 was made and when treated with base [lithium bis(trimethylsilyl)amide] its anion became THF soluble whereby it reacted readily with 2-(sulfinylamino)benzoyl chloride 549 to give luotonin A in 85% yield.213
Neoamphimidine A 550, a pyridoacridine alkaloid, has been extracted from the sponge Xestospongia, species unknown. This metabolite has the unusual property of inducing topisomerase 11 to catenate with DNA.214
Of three new alkaloids isolated from the dark purple coloured marine tunicate Cystodytes violatinctus, two belong to the shemilamine group; they are shemilamine D 551 and shemilamine E 552. The other has a new heterocyclic ring system 553 and is called tintamine.215
The proposed biosynthesis of the manzamine alkaloid group involves the intramolecular cyclisation of an amine such as 554. This has now been realised by treating 554 successively with perchlorobenzoic acid, then trifluoroacetic anhydride, followed by sodium borohydride reduction. A low yield (0.3%) of keramaphidin B 555 was produced.216
The first synthesis of ircinal A 556, ircinol A 557, manzamine A 558 and manzamine D 559 has been reported. Control of stereochemistry was maintained throughout the synthesis mostly through the photochemical cascade employed in the synthesis![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 217 of the parent heterocycle ircinal A 556. Starting with secondary amine 560 and acetylenic ketone 561 they gave the requisite vinylogous amide photosubstrate 562 which on photoaddition and retro-Mannich fragmentation led, viaO-closure of ketoiminium intermediate 563, to aminal 564. Exposure of 564 to pyridinium acetate led to a single stereoisomer 565 which closely resembles the manzamine ring system. Manipulation of R1 to Ts and R2 to COOMe, 566, thus allowed ring closure to afford 567. Conversion of the methyl ester group into aldehyde thus resulted in ircinal A 556. Further conversion of ircinal A to ircinol A 557, manzamine A 558 and manzamine D 559 followed.
217 of the parent heterocycle ircinal A 556. Starting with secondary amine 560 and acetylenic ketone 561 they gave the requisite vinylogous amide photosubstrate 562 which on photoaddition and retro-Mannich fragmentation led, viaO-closure of ketoiminium intermediate 563, to aminal 564. Exposure of 564 to pyridinium acetate led to a single stereoisomer 565 which closely resembles the manzamine ring system. Manipulation of R1 to Ts and R2 to COOMe, 566, thus allowed ring closure to afford 567. Conversion of the methyl ester group into aldehyde thus resulted in ircinal A 556. Further conversion of ircinal A to ircinol A 557, manzamine A 558 and manzamine D 559 followed.
The unusual alkaloid psylloborine A has been obtained from the ladybird beetle Psyllobora vigintiduopunctata.218 It comprises, structurally speaking, two azanaphthalene units, the structure of which 568 was determined on 220 μg of material; NMR spectroscopy was utilised to its most sophisticated extent.
A synthesis of (±)-brevianamide B 569 has been reported which suggests that the Diels–Alder cyclisation used may have a biosynthetic counterpart.219
One of seventeen alkaloids isolated from the air-dried leaves of Alstonia villosa is (19Z)-5α-methoxyrhazimine 570. It and four other alkaloids isolated are new.220
Circumdatin A, B and C are three new benzodiazepine alkaloids isolated from the culture fluid of Aspergillus ochraceus.221 Two, namely A 571 and B 572 are zwitterions while C is 573. Cogeners okaramine H 574 and okaramine I 575 are produced by the fungus Aspergillus aculeatus KF-428.222 These two alkaloids did not possess any insecticidal activity against silkworms as opposed to their counterparts A to G.
Renieramycin H 576 and I 577 have been found in the bright-blue coloured sponge Haliclona cribriculis Dendy.223
Anticancer agent Mer-WF-1726 is produced by a Libertella species. It, 578, inhibits the proliferation of human leukaemia cells type K562.224
A study of the biosynthesis of marcfortine A 579, using 13C, 15N labels, has established that lysine is incorporated into the pipecolate moiety, with the terminal ε nitrogen being lost in the process.225
A new alkaloid called asperparaline 580 has been produced by the micro-organism Aspergillus japonicus strain JV-23. It has potential as an agricultural/medical insecticide.226
A Great Barrier Reef ascidian Didemnum chartaceum has yielded up five new lamellarin-class alkaloids.227 They are the 20-sulfate esters of laminarin B 581, laminarin C 582, and laminarin L 583 together with laminarin G-8 sulfate 584 and unsulfated laminarin Z 585. The extraction of a Red Sea marine sponge of the Raspailia family has yielded three novel alkaloids.228 Called asmarines they have been designated A 586, B 587 and an epimer of B namely C 588. Pseudotheonamides are a group of six serine protease inhibitors isolated from the marine sponge Theonella swinhoei.229 Pseudotheonamide A1 is 589, A2 is 590 and B2 is 591. Pseudotheonamide C is 592, while pseudotheonamide D is 593 and the sixth metabolite is dihydrocyclotheonamide A 594.
References
- Y.-R. Bi, K.-H. Yung and Y.-S. Yum, Plant Sci. (Shannon Irel.), 1998, 135, 103 (Chem. Abstr., 1999, 129, 200568) Search PubMed.
- S. Yui, M. Mikami, M. Kitahara, Y. Mikio and M. Yamazaki, Immunopharmacology, 1998, 40, 151 (Chem. Abstr., 1999, 130, 75906) CrossRef CAS.
- M. T. Smith, C. R. Field, N. R. Couch and M. Hirst, Pharm. Biol., 1998, 36, 173 (Chem. Abstr., 1999, 130, 293955) Search PubMed.
- T. B. K. Lee, K. E. Goehiung and Z. Ma, J. Org. Chem., 1998, 63, 4535 CrossRef CAS.
- G. A. Potter and P. D. Tiffen , PCT Int. Appl WO 98 46,610 (Chem. Abstr., 1998, 129, 302746). .
- F. Viladomat, J. Bastida, C. Codina, X. Solans and M. Font-Bardia, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1999, 55, 385 CrossRef.
- L. H. Pham, W. Dopke, J. Wagner and C. Mugge, Phytochemistry, 1998, 48, 371 CrossRef CAS.
- M. A. Ramadan, Bull. Pharm. Sci., 1998, 21, 97 (Chem. Abstr., 1999, 130, 293960) Search PubMed.
- C. K. Tran, Tap Chi Duoc Hoc., 1998, 11 (Chem. Abstr., 1999, 130, 158320) Search PubMed.
- J. J. Nair, W. E. Campbell, D. W. Gammon, F. Albrecht, F. Viladomat, C. Codina and J. Bastida, Phytochemistry, 1998, 49, 2539 CrossRef CAS.
- S. Ide, B. Sener and S. Ozturk, J. Chem. Crystallogr., 1998, 288, 577 (Chem. Abstr., 1999, 130, 182646) CrossRef.
- J. Bastida, Planta Med., 1998, 648, 679 (Chem. Abstr., 1999, 129, 293754).
- S. Noyan, G. H. Reutsch, M. A. Onur, T. Gozler, B. Gozler and M. Hesse, Heterocycles, 1998, 488, 1777 (Chem. Abstr., 1999, 130, 22839) Search PubMed.
- N. Unver, T. Gozler, N. Walch, B. Gozler and M. Hesse, Phytochemistry, 1999, 50, 1255 CrossRef CAS.
- A. Linden, G. Akineri, S. Noyan, T. Gozler and M. Hesse, Acta Crystallogr., Sect. C, Cryst. Struct. Commun., 1998, 54, 1653 CrossRef.
- C. E. Seaforth, G. W. Williams and W. F. Tinto, Fitoterapia, 1998, 698, 79 (Chem. Abstr., 1999, 129, 38779).
- J. Bastida, F. Viladomat and C. Codina, Stud. Nat. Prod. Chem., 1998, 208, 323 (Chem. Abstr., 1999, 130, 196786) Search PubMed.
- J. Labrana, G. Choy, X. Solans, M. Font-Bardia, G. De la Fuente, F. Viladomat, C. Codina and J. Bastida, Phytochemistry, 1998, 50, 183 CrossRef.
- M. Selles, F. Viladomat, J. Bastida and C. Codina, Plant Cell Rep., 1999, 188, 646 (Chem. Abstr., 1999, 130, 249594) CrossRef.
- B. Sener, S. Konukol, C. Kruk and U. K. Pandit, Nat. Prod. Sci., 1998, 48, 149 (Chem. Abstr., 1999, 130, 63659) Search PubMed.
- D. T. A. Youssef and A. W. Frahm, Planta Med., 1998, 648, 669 (Chem. Abstr., 1999, 129, 293753).
- K. Kojima, M. Mutsuga, M. Inoue and Y. Ogihara, Phytochemistry, 1998, 48, 1199 CrossRef CAS.
- T. Kamikubo and K. Ogasawara, Chem. Commun., 1998, 783 RSC.
- O. Yamada and K. Ogasawara, Tetrahedron Lett., 1998, 39, 7747 CrossRef CAS.
- Y. Kita , T. Takata and M. Arisawa , Jpn. Kokai Tokkyo Koho, JP 1180, 170, 1999 (Chem. Abstr., 1999, 130, 252525). .
- Y. Kita, M. Arisawa, M. Gyoten, M. Nakajima, R. Hamada, H. Tohina and T. Takada, J. Org. Chem., 1998, 63, 6625 CrossRef CAS.
- J. Cossy, L. Tresnard and D. G. Pardo, Tetrahedron Lett., 1999, 40, 1125 CrossRef CAS.
- G. E. Keck, S. F. McHardy and J. A. Murray, J. Am. Chem. Soc., 1995, 117, 7289 CrossRef CAS.
- G. E. Keck, T. T. Wager and S. F. McHardy, J. Org. Chem., 1998, 63, 9164 CrossRef CAS.
- P. Magnus and I. K. Sebhat, Tetrahedron, 1998, 54, 15509 CrossRef CAS.
- W. H. Pearson and B. W. Lian, Angew. Chem., Int. Ed., 1998, 37, 1724 CrossRef CAS.
- T. Nakanishi, M. Susuki, A. Mashiba, K. Ishikawa and T. Yokotsuka, J. Org. Chem., 1998, 63, 4235 CrossRef CAS.
- G. R. Green, I. S. Mann and M. V. Mullane, Tetrahedron, 1998, 54, 9875 CrossRef.
- T. Harayama, T. Akiyama, Y. Nakano and K. Shibaike, Heterocycles, 1998, 48, 1989 (Chem. Abstr., 1998, 130, 125238) Search PubMed.
- J. Khalafy, R. H. Prager and C. H. Williams, Aust. J. Chem., 1999, 52, 31 CAS.
- A. Nishida, M. Fuwa, Y. Fijikawa, E. Nakahata, A. Furunno and M. Nakagawa, Tetrahedron Lett., 1998, 39, 5983 CrossRef CAS.
- S. Takashi, N. Tuchiya, A. Sato, T. Negishi, Y. Takamatsu, Y. Matsushita, T. Watanabe, Y. Iijima, H. Haruyama, T. Kinoshita, M. Tohru and K. Kodama, J. Antibiot., 1998, 51, 805 (Chem. Abstr., 1998, 129, 341517) Search PubMed.
- C. J. Forsyth, F. Ahmed, R. D. Cink and C. S. Lee, J. Am. Chem. Soc., 1998, 120, 5597 CrossRef CAS.
- C. S. Lee and C. J. Forsyth, Tetrahedron Lett., 1996, 37, 6449 CrossRef CAS.
- R. D. Cink and C. J. Forsyth, J. Org. Chem., 1997, 62, 5672 CrossRef CAS.
- F. Ahmed and C. J. Forsyth, Tetrahedron Lett., 1998, 39, 183 CrossRef CAS.
- S. Matsunaga, T. Sugawara and N. Fusetani, J. Nat. Prod., 1998, 61, 1164 CrossRef CAS.
- X. Fu, F. J. Schmits, M. Kelly-Borges, T. L. McCready and C. F. B. Holmes, J. Org. Chem., 1998, 63, 7957 CrossRef CAS.
- P. Ciminullo, C. Dell’Aversano, E. Fattorusso, S. Magno and M. Pansini, J. Nat. Prod., 1999, 62, 590 CrossRef CAS.
- C. J. Moody and M. C. Bagley, Chem. Commun., 1998, 2049 RSC.
- M. C. Norley and G. Pattenden, Tetrahedron Lett., 1998, 39, 3087 CrossRef CAS.
- D. J. Freeman and G. Pattenden, Tetrahedron Lett., 1998, 39, 3251 CrossRef CAS.
- R. Benhida, R. Lezama and J. L. Fourrey, Tetrahedron Lett., 1998, 39, 5963 CrossRef CAS.
- F. Cafieri, E. Fatturusso and O. Terglialetela-Scafati, J. Nat. Prod., 1998, 61, 1171 CrossRef CAS.
- T. V. Hughes and N. P. Cava, Tetrahedron Lett., 1998, 39, 9629 CrossRef CAS.
- J. Shin, Y. Seo, K. W. Cho, J.-R. Rho and C. J. Sim, J. Nat. Prod., 1999, 62, 647 CrossRef CAS.
- I. Kawasaki, H. Katsumo, Y. Nakayama, M. Yamashita and S. Ohta, Heterocycles, 1998, 48, 1887 (Chem. Abstr., 1999, 130, 25218) Search PubMed.
- R. W. Schumacher and B. S. Davidson, Tetrahedron, 1999, 55, 935 CrossRef CAS.
- M. Ohba, Y. Nishimura, M. Imasho, T. Fujii, J. Kubanek and R. J. Anderson, Tetrahedron Lett., 1998, 39, 5999 CrossRef CAS.
- U. H. Maier and M. H. Zenk, Phytochemistry, 1998, 49, 1791 CrossRef CAS.
- X. Shen, T. L. Perry, C. D. Dunbar, M. Kelly-Borges and M. T. Hamann, J. Nat. Prod., 1998, 61, 1302 CrossRef CAS.
- S. Urban, P. de A. Leone, A. R. Carroll, G. A. Fechner, J. Smith, J. N. A. Hooper and R. J. Quinn, J. Org. Chem., 1999, 64, 731 CrossRef CAS.
- K. C. Nicolaou, M. R. V. Finlay, S. Ninkovic and F. Sarabia, Tetrahedron, 1998, 54, 7127 CrossRef CAS.
- S. C. Sinha, C. F. Barbas III and R. A. Lerner, Proc. Natl. Acad. Sci. USA, 1998, 95, 14603 (Chem. Abstr., 1999, 130, 153488) CrossRef CAS.
- N. Sitachichitta, J. Rossi, M. A. Roberts, W. H. Gerwick, M. D. Fletcher and C. L. Willis, J. Am. Chem. Soc., 1998, 120, 7131 CrossRef CAS.
- J. C. Muir, G. Pattenden and T. Ye, Tetrahedron Lett., 1998, 39, 2861 CrossRef CAS.
- D. Klein, J. C. Braekman, D. Daloze, L. Hoffmann, G. Castillo and V. Demoulin, Tetrahedron Lett., 1999, 40, 695 CrossRef CAS.
- R. G. Pettit, G. R. Pettit and K. C. Hazen, Antimicrob. Agents Chemother., 1998, 42, 2961 (Chem. Abstr., 1999, 130, 63554) CAS.
- R. Domo, R. M. Rzasa, H. A. Shea, K. Park, J. M. Langenhan, L. Sun, A. Akhiezer and J. O. Lniu, J. Am. Chem. Soc., 1998, 120, 12237 CrossRef.
- H. Zhang, H. Tomoda, N. Tabata, M. Oohori, M. Shinose, Y. Takahashi and S. Omura, J. Antibiot., 1999, 52, 29 (Chem. Abstr., 1999, 130, 249209) Search PubMed.
- X. Fu, T. Do, F. J. Schmitz, A. Andrusevich and M. H. Engel, J. Nat. Prod., 1998, 61, 1547 CrossRef CAS.
- S. Fudou , S. Yamanaka , T. Yoshimura , H. Oga and Y. Sakagami , Jpn. Kokai Tokkyo Koho, JP 10 204,068, 1998 (Chem. Abstr., 1998, 129, 174724). .
- M. Ojika, Y. Suzuki, A. Tsukamoto, Y. Sakagami, R. Fundou, T. Yoshimura and S. Yamanaka, J. Antibiot., 1998, 51, 275 (Chem. Abstr., 1998, 129, 2483) Search PubMed.
- Y. Suzuki, M. F. Ojika, Y. Sakagami, R. Fudou and S. Yamanaka, Tetrahedron, 1998, 54, 11399 CrossRef CAS.
- S. Kobayashi, H. Nakai, Y. Ikenishi, W.-Y. Sun, M. Ozaki, Y. Hayase and R. Takeda, J. Antibiot., 1998, 51, 328 (Chem. Abstr., 1998, 129, 2490) Search PubMed.
- J. W. Ahn, S.-H. Woo, C.-O. Lee, K.-Y. Cho and B.-S. Kim, J. Nat. Prod., 1999, 62, 495 CrossRef CAS.
- K. Kikuchi , C. Chen , A. Lung , K. Adachi , M. Nishishima , F. Nishida , T. Takadera and H. Sano , Jpn. Kokai Tokkyo Koho, JP 10 101,657, 1998 (Chem. Abstr., 1998, 129, 3913). .
- K. Kikuchi , Y. Chen , K. Adachi , M. Nishijima , A. Nishida , T. Takatera and H. Sano , Jpn. Kokai Tokkyo Koho JP 10 245,377, 1998 (Chem. Abstr., 1998, 129, 244217). .
- K. Okumura, A. Ito and D. Yoshioka and Ch-gi. Shin, Heterocycles, 1998, 48, 1319 (Chem. Abstr., 1998, 129, 260841) Search PubMed.
- T. Sasaki, T. Otani, H. Matsumoto, N. Unemi, M. Hamada, T. Takeuchi and M. Hori, J. Antibiot., 1998, 51, 715 (Chem. Abstr., 1998, 129, 272880) Search PubMed.
- D. Kaukshik, M. P. Dobhal, B. C. Joshi, R. P. Tyagi and G. S. Negi, Himalayan Chem. Pharm. Bull., 1998, 15, 22 (Chem. Abstr., 1999, 130, 12350) Search PubMed.
- Y.-S. Bahn, J.-M. Park, D.-H. Bai, S. Takase and J.-H. Yu, J. Antibiot., 1998, 51, 902 (Chem. Abstr., 1999, 130, 63418) Search PubMed.
- S. Sperry and P. Crews, J. Nat. Prod., 1998, 61, 859 CrossRef CAS.
- A. G. Soman, J. B. Gloer and D. T. Wicklow, J. Nat. Prod., 1999, 62, 386 CrossRef CAS.
- L. D. Ismail, P. Lorenz and F. R. Stermitz, J. Nat. Prod., 1998, 61, 1174 CrossRef CAS.
- R. F. Salm, H. D. Zinsmeister and T. Eicher, Phytochemistry, 1998, 49, 887 CrossRef CAS.
- M. Ueda, Y. Sawai, Y. Shibazaki, C. Tashiro, T. Ohnuki and S. Yamamura, Biosci., Biotechnol., Biochem., 1998, 62, 2133 (Chem. Abstr., 1999, 130, 150931) Search PubMed.
- N. K. Kalia, B. Singh and R. P. Sood, J. Nat. Prod., 1999, 62, 311 CrossRef CAS.
- H.-J. Yu, C.-C. Chen and B.-J. Shieh, J. Nat. Prod., 1998, 61, 1017 CrossRef CAS.
- Z. Latif, T. C. Hartley, M. J. Rice, R. D. Waigh and P. G. Waterman, J. Nat. Prod., 1998, 61, 614 CrossRef CAS.
- J. W. Kim, K. Shin-ya, K. Furihata, Y. Hayakawa and H. Seto, J. Org. Chem., 1999, 64, 153 CrossRef CAS.
- B. Kunze, R. Jansen, F. Sasse, G. Hofle and H. Richenback, J. Antibiot., 1998, 51, 1075 (Chem. Abstr., 1999, 130, 194051) Search PubMed.
- K. Shibata, M. Hanafi, J. Fujii, O. Sakanaka, K. Linuma, M. Ucki and M. Taniguchi, J. Antibiot., 1998, 51, 1113 (Chem. Abstr., 1999, 130, 168617) Search PubMed.
- Y. Watanabe , M. Kurotaki , M. Nishide and H. Yoshida , Jpn. Kokai Tokkyo Koho JP 11 49,768, 1999 (Chem. Abstr., 1999, 130, 337075). .
- S. P. B. Ovenden, R. J. Capon, E. Lacey, J. H. Gill, T. Friedel and D. Wadsworth, J. Org. Chem., 1999, 64, 1140 CrossRef CAS.
- K. Akaji, Y. Hayashi, Y. Kiso and N. Kurijama, J. Org. Chem., 1999, 64, 405 CrossRef CAS.
- G. G. Harrigan, H. Luesch, W. Y. Yoshida, R. E. Moore, D. G. Nagle, V. J. Paul, S. L. Mooberry, T. H. Corbett and F. A. Valeriote, J. Nat. Prod., 1998, 61, 1075 CrossRef CAS.
- H. Seto , K. Shin-ya , T. Yaguchi and T. Sasaki , PCT Int. Appl. WO 98 41,503 (Chem. Abstr., 1998, 129, 274808). .
- K. Yoshikawa , Y. Nakayama , M. Hayashi , T. Unemoto and K. Mochida , J. Antibiot., 1999, 52, 182 (Chem. Abstr., 1999, 130, 322841). .
- A. Sato , T. Horinchi , N. Shimizu , H. Onuki and K. Kimura , Jpn. Kokai Tokkyo Koho JP 10 101,662, 1998 (Chem. Abstr., 1998, 129, 3915). .
- R. E. Mitchell and K. L. Ford, Phytochemistry, 1998, 49, 1579 CrossRef CAS.
- R. J. Broadbridge, R. P. Sharma and M. Akhtar, Chem. Commun., 1998, 1449 RSC.
- M. Shiozaki, N. Deguchi, T. Mochizuki, T. Wakabayasahi, T. Ishikawa, H. Haruyama, Y. Kawai and M. Nishijima, Tetrahedron, 1998, 544, 11861 CrossRef.
- K. Ishida, H. Matsuda and M. Murakami, Tetrahedron, 1998, 54, 13475 CrossRef CAS.
- D. Lontsi, M. T. Martin, M. Litaudon, T. Sevenet and M. Pais, J. Nat. Prod., 1998, 61, 953 CrossRef CAS.
- T. Mukhopadhyay, R. G. Bhat, K. Roy, E. K. S. Vijayakumar and B. D. Ganguli, J. Antibiot., 1998, 51, 439 (Chem. Abstr., 1998, 129, 38538) Search PubMed.
- G. Macdonald, L. Alcaraz, N. J. Lewis and R. J. K. Taylor, Tetrahedron Lett., 1998, 39, 5433 CrossRef CAS.
- H. Toshima, K. Maru, Y. Jiao, T. Yoshihara and A. Ichihara, Tetrahedron Lett., 1999, 40, 935 CrossRef CAS.
- H. Toshima, K. Maru, M. Saito and A. Ichihara, Tetrahedron Lett., 1999, 40, 939 CrossRef CAS.
- S. Kodani, R. Ishida and H. Murakami, J. Nat. Prod., 1998, 61, 1046 CrossRef CAS.
- T. S. Sugawara, A. Tanaka, K. Tanajka, K. Nagai, K. Suzuki and T. Suzuki, J. Antibiot., 1998, 51, 435 (Chem. Abstr., 1998, 129, 78913) Search PubMed.
- N. U. Sata, M. Sugano, S. Matsunaga and N. Fusetani, Tetrahedron Lett., 1999, 40, 719 CrossRef CAS.
- H. Kakega, M. Morishita, A. Ikeno, K. Kobinata, Y. Tatsuya and H. Osada, J. Antibiot., 1998, 51, 963 (Chem. Abstr., 1999, 130, 78491) Search PubMed.
- K. Hunterding, P. Hagenbuch, J. Retey and H. Waldmann, Angew. Chem., Int. Ed., 1998, 37, 1236 CrossRef.
- L.-M. Zeng, Y. Zhu, S.-J. Yan and J.-Y. Su, Chem. Res. Chin. Univ., 1998, 14, 426 (Chem. Abstr., 1999, 130, 309248) Search PubMed.
- U. Waspi, D. Blanc, T. Winkler, P. Ruedi and R. Dudler, Mol. Plant–Microbe Interact., 1998, 11, 727 (Chem. Abstr., 1998, 129, 200217) Search PubMed.
- M. Igarashi , H. Naganawa , M. Hamata and T. Takeuchi , Jpn. Kokai Tokkyo Koho JP 11 103,886, 1999 (Chem. Abstr., 1999, 130, 337076). .
- F. Yokokawa and T. Shioiri, J. Org. Chem., 1998, 63, 8638 CrossRef CAS.
- J. D. White, R. Hanselmann and D. J. Wardrop, J. Am. Chem. Soc., 1999, 121, 1106 CrossRef CAS.
- M.-A. Bae, K. Yamada, D. Uemura, J. H. Seu and Y. H. Kim, J. Microbiol. Biotechnol., 1998, 8, 455 (Chem. Abstr., 1999, 130, 179712) Search PubMed.
- H. Nishioka , S. Nakajima , M. Nagashima , K. Kojiri and H. Suda , Jpn. Kokai Tokkyo Koho JP 10, 147,594, 1998 (Chem. Abstr., 1998, 129, 66909). .
- W. F. Tinto, A. J. Lough, S. McLean, W. F. Reynolds and M. Yu, Tetrahedron, 1998, 54, 4451 CrossRef CAS.
- K. K. Schumacher, D. B. Hauze, J. Jiang, J. Szewczyk, R. E. Reddy, F. A. Davis and M. M. Joullie, Tetrahedron Lett., 1999, 40, 455 CrossRef CAS.
- P. Raman, S. S. Stokes, Y. M. Angell, G. R. Flentke and D. H. Rich, J. Org. Chem., 1998, 63, 5734 CrossRef CAS.
- Y.-C. Wang, N.-H. Tan, J. Zhou and H.-H. Wu, Phytochemistry, 1998, 49, 1453 CrossRef CAS.
- T. Lloyd-Williams, G. Jou, I. Gonzalez, J. M. Caba, F. Albericio and E. Giralt, Pept. Proc. Eur. Pept. Symp. 24th, 1996 (Pub 1998) 589 (Chem. Abstr. 1999, 130, 14200). Search PubMed.
- A. Zampella, C. Giannini, C. Debitus, C. Roussakis and M. V. D’Auria, J. Nat. Prod., 1999, 62, 332 CrossRef CAS.
- P. A. Grieco and M. Reilly, Tetrahedron Lett., 1998, 39, 8925 CrossRef CAS.
- T. Okana, T. Sano and K. Kaya, Tetrahedron Lett., 1999, 40, 2379 CrossRef.
- T. Onuki , N. Sugita , O. Kono , Y. Koguchi , T. Murakami and M. Nishio , Jpn. Kokai Tokkyo Koho JP 11, 29,595, 1999 (Chem. Abstr., 1999, 130, 181555). .
- K. Ooka, M. Tanaka, T. Ishikawa and F. Kato, Biol. Pharm. Bull, 1999, 22, 107 (Chem. Abstr., 1999, 130, 249191) Search PubMed.
- H. H. Wasserman, J.-H. Chen and M. Xia, J. Am. Chem. Soc., 1999, 121, 1401 CrossRef CAS.
- A. J. Fujiwara and Y. Abe , Jpn. Kokai Tokkyo Koho JP 10 175,996, 1998 (Chem.Abstr., 1998, 129, 135262). .
- T. Takeuchi , N. Matsumoto , I. Momose , H. Iinuma , H. Naganawa , I. Sawa and M. Hamada , Jpn. Kokai Tokkyo Koho JP 10 287,681, 1998 (Chem. Abstr., 1999, 130, 3112). .
- K. S. Ohda , Y. Takebayashi , K. Nagai , T. Yokoi , T. Nanya , S. Kuromitsu and N. Shindo , PCT Int. Appl. WO 98 45,320 (Chem. Abstr., 1998, 129, 315073). .
- D. L. Boger, M. W. Ledeboer and M. Kume, J. Am. Chem. Soc., 1999, 121, 1098 CrossRef CAS.
- P. S. Moore, J. A. Trischman, D. Seng, D. Kho, P. R. Jensen and W. Fenical, J. Org. Chem., 1999, 64, 1145 CrossRef.
- V. R. Hegde, M. S. Puar, T. M. Chan, P. Dai, P. R. Das and M. Patel, J. Org. Chem., 1998, 63, 9584 CrossRef CAS.
- R. Kanasaki , S. Takase , M. Hashimoto , N. Shiraishi , H. Ohki and K. Kawabata , PCT Int. Appl. WO 98 22,498 (Chem. Abstr., 1998, 129, 53437). .
- G. G. Harrkigan, W. Y. Yoshida, R. E. Moore, D. G. Nagle, P. U. Park, J. Biggs, V. J. Paul, S. L. Mosberry, T. H. Corbett and F. A. Valeriote, J. Nat. Prod., 1998, 61, 1221 CrossRef.
- D. P. Dale, J. Carroll, S. Naylor and P. Crews, J. Org. Chem., 1998, 63, 8757 CrossRef.
- T. Sano, K. A. Beattie, G. A. Codd and K. Kaya, J. Nat. Prod., 1998, 61, 851 CrossRef CAS.
- T. M. Kamenecka and S. J. Danishefsky, Angew. Chem., Int. Ed., 1998, 37, 2993 CrossRef CAS.
- T. M. Kamenecka and S. J. Danishefsky, Angew. Chem., Int. Ed., 1998, 37, 2995 CrossRef CAS.
- M. V. Redi, M. K. Harper and D. J. Faulkner, Tetrahedron, 1998, 54, 10649 CrossRef.
- T. Laib and J. Zhu, Tetrahedron Lett., 1999, 40, 83 CrossRef CAS.
- S. P. East, F. Shao, L. Williams and M. M. Joullie, Tetrahedron, 1998, 54, 13371 CrossRef CAS.
- G. Helynck, C. Dubertret, D. Frechet and J. Leboul, J. Antibiot., 1998, 51, 512 (Chem. Abstr., 1998, 129, 78935) Search PubMed.
- K. C. Nicolaou, S. Natarajan, H. Li, N. F. Jain, R. Hughes, M. E. Solomon, J. M. Ramanjulu, C. N. C. Boddy and M. Tokayanagi, Angew. Chem., Int. Ed., 1998, 37, 2708 CrossRef CAS.
- K. C. Nicolaou, N. F. Jain, S. Natarajan, R. Hughes, M. E. Solomon, H. Li, J. M. Ramanjuju, M. Takayanagi, A. E. Koumbis and T. Bando, Angew. Chem., Int. Ed., 1998, 37, 2714 CrossRef CAS.
- K. C. Nicolaou, M. Takayanagi, N. F. Jain, S. Natarajan, A. E. Koumbis, T. Bando and J. H. Kamanjulu, Angew. Chem., Int. Ed., 1998, 37, 2717 CrossRef CAS.
- D. A. Evans, M. R. Wood, B. W. Trotter, T. I. Richardson, J. C. Barrow and J. L. Katz, Angew. Chem., Int. Ed., 1998, 39, 2700 CrossRef CAS.
- D. A. Evans, C. J. Dunsmore, P. S. Watson, M. R. Wood, T. I. Richardson, B. W. Trotter and J. L. Katz, Angew. Chem., Int. Ed., 1998, 37, 2704 CrossRef CAS.
- M. Ge, C. Thompson and D. Kahne, J. Am. Chem. Soc., 1998, 120, 11014 CrossRef CAS.
- D.-G. Liu and G.-Q. Lin, Tetrahedron Lett., 1999, 40, 337 CrossRef CAS.
- W. Y. Yoshida and P. J. Scheuer, Heterocycles, 1998, 47, 1023 (Chem. Abstr., 1998, 129, 2507) Search PubMed.
- T. Ohta, H. Inoue, G. Kusano and Y. Oshima, Heterocycles, 1998, 47, 883 (Chem. Abstr., 1998, 129, 2506) Search PubMed.
- M. Ueda, M. Asano and S. Yamamura, Tetrahedron Lett., 1998, 39, 9731 CrossRef CAS.
- Y. Venkateswarlu, M. R. Rao and U. Venkatesham, J. Nat. Prod., 1998, 61, 1388 CrossRef CAS.
- H. Miyabe, M. Torieda, K. Inoue, K. Tajiri, T. Kiguchi and T. Naito, J. Org. Chem., 1998, 63, 4397 CrossRef CAS.
- K. M. Jenkins, S. G. Toske, P. R. Jensen and W. Fenical, Phytochemistry, 1998, 49, 2299 CrossRef CAS.
- S. Omura, Y. Enomoto, M. Shinose, Y. Takahashi, Y. Iwai and K. Shiomi, J. Antibiot., 1999, 52, 61 (Chem. Abstr., 1999, 130, 236505) Search PubMed.
- F. Schroder and W. Francke, Tetrahedron, 1998, 54, 5259 CrossRef CAS.
- J. Breinholt, H. C. Jensen, A. Kjaer, C. Olsen, B. R. Rassing, C. N. Rosendahl and I. Sotofte, Acta. Chem. Scand., 1998, 52, 631 (Chem. Abstr., 1998, 129, 38551) Search PubMed.
- S. Kodani, K. Ishida and M. Murakami, J. Nat. Prod., 1998, 61, 854 CrossRef CAS.
- M. Ueda and S. Yamamura, Tetrahedron Lett., 1999, 40, 353 CrossRef CAS.
- Y. Cui and W. Li, Bopuxue Zazhi, 1998, 15, 515 (Chem. Abstr., 1999, 130, 194363) Search PubMed.
- T. Akiyama, R. Sawa, H. Nagamawa, Y. Muraoka, T. Ayogi and T. Takeuchi, J. Antibiot., 1998, 51, 372 (Chem. Abstr., 1998, 129, 2491) Search PubMed.
- S. B. Singh, D. L. Zink, H. A. Goetz, A. W. Dombrowski, J. D. Polishook and D. J. Hazuda, Tetrahedron Lett., 1998, 39, 2243 CrossRef CAS.
- L. Vertesy, W. Aretz, E. Ehlers, S. Hawser, D. Isert, M. Knauf, H. Kurz, M. Scheill, M. Vogel and J. Wink, J. Antibiot., 1998, 51, 921 (Chem. Abstr., 1999, 130, 49628) Search PubMed.
- Y. Merimoto, C. Yokoe, H. Kurihara and T. Kinoshita, Tetrahedron, 1998, 54, 12197 CrossRef CAS.
- K. Drandorov, A. Guggisberg, A. Linden and M. Hesse, Helv. Chim. Acta, 1998, 81, 1773 CrossRef.
- G. Karigiannis, P. Mamos, G. Balayiannis, I. Katsoulis and D. Papaioannou, Tetrahedron Lett., 1998, 39, 5117 CrossRef CAS.
- K. Torikoshi , T. Yamauchi , S. Nakajima , M. Kamiya , M. Nagashima , K. Kojiri and H. Suda , Jpn. Kokai Tokkyo Koho JP 11 71,379, 1999 (Chem. Abstr., 1999, 130, 280924). .
- R. G. S. Berlinck, R. Britton, E. Piers, L. Lim, M. Roberge, R. M. de Rocha and R. J. Anderson, J. Org. Chem., 1998, 63, 9850 CrossRef CAS.
- H. C. Vervoort, W. Fenical and P. A. Keifer, J. Nat. Prod., 1999, 62, 389 CrossRef CAS.
- E. M. Beccalli, M. L. Gelmi and A. Marchesini, Tetrahedron, 1998, 54, 6909 CrossRef CAS.
- K. Watanabe, K. Iguchi and K. Fyujimori, Heterocycles, 1998, 49, 355 (Chem. Abstr., 1999, 130, 180124) Search PubMed.
- Y. Kashman, G. Koren-Goldshlager, M. D. G. Cravalos and M. Schleyer, Tetrahedron Lett., 1999, 40, 997 CrossRef CAS.
- M. S. C. Pedras, K. C. Smith and J. L. Taylor, Phytochemistry, 1998, 49, 1575 CrossRef CAS.
- T. Watanabe, S. Kohzuma, T. Takeuchi, M. Otsuka and K. Umezawa, Chem. Commun., 1998, 1097 RSC.
- K. L. Rinehart , E. A. Jares-Erijman , USP, 5,756,734, 1998 (Chem. Abstr., 1998, 129, 38964). .
- W.-C. Chen and M. M. Joullie, Tetrahedron Lett., 1998, 39, 8401 CrossRef CAS.
- D. E. Williams, K. Bombuwala, E. Lobkovsky, D. D. de Silva, V. Karunaratue, T. A. Allen, J. Clardy and R. J. Andersen, Tetrahedron Lett., 1998, 39, 9579 CrossRef.
- Y.-T. Ma, M.-C. Huang, F.-L. Hsu and H.-F. Chang, Phytochemistry, 1998, 48, 1083 CrossRef CAS.
- S. Kobayashi, M. Ueno, R. Suzuki and H. Ishitani, Tetrahedron Lett., 1999, 40, 2175 CrossRef CAS.
- J. M. Cronan, T. R. Davidson, F. L. Singleton, R. R. Colwell and J. H. Cardellina, Nat. Prod. Lett., 1998, 11, 271 (Chem. Abstr., 1998, 129, 65348) Search PubMed.
- K. Fukumoto , T. Asari , T. Harada , S. Kono , K. Kano , H. Kawashima , H. Sekiya and K. Oomizo , Jpn. Tokai, Tokkyo Koho. JP 10 130,266, 1998 (Chem. Abstr., 1998, 129, 53444). .
- H. Hayashi and A. Sakaguchi, Biosci., Biotechnol., Biochem., 1998, 62, 804 (Chem. Abstr., 1998, 129, 51855) Search PubMed.
- K. M. Werner, J. M. de los Santos and S. M. Weinreb, J. Org. Chem., 1999, 64, 686 CrossRef CAS.
- K. Watanabe, K. Igukchi and K. Fukimori, Heterocycles, 1998, 49, 269 (Chem. Abstr., 1999, 130, 180123) Search PubMed.
- K.-J. Kim and T. K. Kim, Yakhak Hoechi, 1998, 42, 552 (Chem. Abstr., 1999, 130, 121939) Search PubMed.
- T. Ross Kelly, Y. Fu and R. L. Xie, Tetrahedron Lett., 1999, 40, 1857 CrossRef.
- M. Tsuboi, S. Shimizu, M. Yamashita, Y. Tsurumi and S. Hashimoto, PCT Int. Appl. WO 98 18,953 (Chem. Abstr., 1998, 129, 3903). .
- J. Breinholt, H. Gurtler, A. Kjaer, S. E. Nielsen and C. E. Olsen, Acta Chem. Scand., 1998, 52, 1040 Search PubMed.
- B. D. Morris and M. R. Prinsep, J. Org. Chem., 1998, 63, 9545 CrossRef CAS.
- Y.-L. Leu, Y.-Y. Chan and T.-S. Wu, Phytochemistry, 1998, 48, 743 CrossRef.
- T.-S. Wu, Y.-L. Yann and Y.-Y. Chan, Chem. Pharm. Bull., 1998, 46, 1624 (Chem. Abstr., 1999, 130, 49821) Search PubMed.
- E. Olson , S. J. Trew , S. K. Wrigley , L. Pairet , M. A. Hayes , S. Martin and D. A. Kau , PCT Int. Appl.WO 98 25,931 (Chem. Abstr., 1998, 129, 52003). .
- D. L. Boger, J. Hong, M. Hikota and M. Ishida, J. Am. Chem. Soc., 1999, 121, 2471 CrossRef CAS.
- M. Iwao, O. Motoi, T. Fukuda and F. Ishibashi, Tetrahedron, 1998, 54, 8999 CrossRef CAS.
- G. A. Kraus and N. Selvakumar, J. Org. Chem., 1998, 63, 9846 CrossRef CAS.
- Y. Kila, M. Egi and H. Tohma, Chem. Commun., 1999, 143 RSC.
- Y. Moro-oka, T. Fukuda and M. Iwao, Tetrahedron Lett., 1999, 40, 1713 CrossRef CAS.
- A. S. Franklin, S. K. Ly, G. H. Mackin, L. E. Overman and A. J. Shaka, J. Org. Chem., 1999, 64, 1512 CrossRef CAS.
- W. F. Tinto, Heterocycles, 1998, 48, 2089 (Chem. Abstr., 1999, 130, 107769) Search PubMed.
- E. Sugino, T. Choshi and S. Hibino, Heterocycles, 1999, 50, 543 (Chem. Abstr., 1999, 130, 153831) Search PubMed.
- L. Boiteau, J. Boivin, A. Liard, B. Quiclet-Sire and S. Z. Zard, Angew. Chem., Int. Ed., 1998, 37, 1128 CrossRef CAS.
- B. K. Joshi, J. B. Gloer, D. T. Wicklow and P. F. Dowd, J. Nat. Prod., 1999, 62, 650 CrossRef CAS.
- T. Sugimori, T. Okawa, S. Eguchi, A. Katchi, E. Yashima and Y. Okamoto, Tetrahedron, 1998, 54, 7997 CrossRef CAS.
- T. Sasaki, I. I. Ohtani, J. Tanaka and T. Higa, Tetrahedron Lett., 1999, 40, 303 CrossRef CAS.
- B. R. Copp, J. Jompa, A. Tahir and C. M. Ireland, J. Org. Chem., 1998, 63, 8024 CrossRef CAS.
- M. K. Aubart and C. H. Heathcock, J. Org. Chem., 1999, 64, 16 CrossRef CAS.
- S. P. Gunasekera, P. J. McCarthy, R. E. Longley, S. A. Pomponi, A. E. Wright, E. Lobkovsky and J. Clardy, J. Nat. Prod., 1999, 62, 173 CrossRef CAS.
- M.-G. Dikoux, W. R. Gamble, Y. E. Hallock, J. H. Cordellina, R. Van Soest and R. Boyd, J. Nat. Prod., 1999, 62, 636 CrossRef.
- Y. Kitahara, F. Tamura, M. Niohimura and A. Kubo, Tetrahedron, 1998, 54, 8421 CrossRef CAS.
- S. Nakahara, J. Matsui and A. Kubo, Tetrahedron Lett., 1998, 39, 5521 CrossRef CAS.
- H. Wang and A. Ganesan, Tetrahedron Lett., 1998, 39, 9097 CrossRef CAS.
- F. S. de Guzman, B. Carte, N. Troupe, D. J. Faulkner, M. K. Harper, G. P. Concepcion, C. Mangalindan, S. S. Matsumoto, L. R. Barrows and G. M. Ireland, J. Org. Chem., 1999, 64, 1400 CrossRef CAS.
- G. Koren-Goldshlager, M. Aknin, E. M. Gaydon and Y. Kashman, J. Org. Chem., 1998, 63, 4601 CrossRef CAS.
- J. E. Baldwin, T. D. W. Claridge, A. J. Culshaw, F. A. Heupel, V. Lee, D. R. Spring, R. Whitehead, R. J. Broughtflower, I. M. Hutton and R. J. Upjohn, Angew. Chem., Int. Ed., 1998, 37, 2661 CrossRef CAS.
- J. D. Winkler and J. M. Axten, J. Am. Chem. Soc., 1998, 120, 6425 CrossRef CAS.
- F. C. Schroder and T. Tolasch, Tetrahedron, 1998, 54, 12243 CrossRef CAS.
- R. M. Williams, J. F. Sanz-Ceruera, F. Sancenon, J. A. Marco and K. M. Halligan, Bioorg. Med. Chem., 1998, 6, 1233 (Chem. Abstr., 1998, 129, 316418) CrossRef CAS.
- F. Abe, T. Yamauchi, H. Shibuya, I. Kitagawa and M. Yamashita, Chem. Pharm. Bull., 1998, 46, 1235 (Chem. Abstr., 1998, 129, 287807) Search PubMed.
- L. Rahbaek, J. Breinholt, J. C. Frisvada and C. Christopherson, J. Org. Chem., 1999, 64, 1689 CrossRef.
- H. Hayashi, K. Furutsuka and Y. Shiona, J. Nat. Prod., 1999, 62, 315 CrossRef CAS.
- P. S. Parameswaran, C. G. Naik, S. Y. Kamat and B. N. Pramanik, Indian J. Chem. Sect. B. Org. Chem. Incl. Med. Chem., 1998, 37, 1258 (Chem. Abstr., 1999, 130, 335476) Search PubMed.
- R. Tsuji , K. Wakazono , K. Isshiki , Y. Watanabe , K. Tsuchihashi and T. Yoshioka , Jpn. Kokai, Tokkyo Koho JP 10 114,776 (Chem. Abstr., 1998, 129, 15357 .
- M. S. Kuo, D. A. Yurek, S. A. Mizsak, J. I. Cialdella, L. Baxzynskyj and V. P. Marshall, J. Am. Chem. Soc., 1999, 121, 1763 CrossRef CAS.
- H. Hayashi and H. Kato , Jpn. Kokai, Tokkyo JP 10 245,383, 1998 (Chem. Abstr., 1998, 129, 244218). .
- R. A. Davis, A. R. Carroll, G. K. Pierens and R. J. Quinn, J. Nat. Prod., 1999, 62, 419 CrossRef CAS.
- T. Yosief, A. Rudi, Z. Stein, I. Goldberg, G. M. D. Gravalos, M. Schleyer and Y. Kashman, Tetrahedron Lett., 1998, 39, 3323 CrossRef CAS.
- Y. Nakao, A. Masuda, S. Matsunaga and N. Fusetani, J. Am. Chem. Soc., 1999, 121, 2425 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2001 |