An efficient synthesis of 5-hydroxy-2(5H)-furanone using a titanium silicate molecular sieve catalyst
Pradeep Kumar* and Rajesh Kumar Pandey
Division of Organic Chemistry: Technology, National Chemical Laboratory, Pune-, 411008, India.. E-mail: tripathi@dalton.ncl.res.in
First published on UnassignedUnassigned8th February 2000
Green ContextThe selective oxidation of simple precursors is one of the fundamental operations in chemistry. Many methods for these transformations are known, but are often associated with large quantities of transition metal waste. This paper demonstrates a highly selective and clean functionalisation of furan using titanium silicate TS-1, one of the most efficient solid catalysts for the selective oxidation of small molecules (channel diameter ca. 0.5 nm), and hydrogen peroxide, a cheap and clean oxidant. The product is a versatile intermediate in the synthesis of several bioactive compounds.DJM |
Summary
Titanium silicate molecular sieve, having MFI (TS-1) topology, efficiently catalyses the oxidation of furan to the corresponding 5-hydroxy-2(5H)-furanone in excellent yields, using dilute hydrogen peroxide (25%) as an oxidising agent; mechanistically the intermediacy of 1O2 is proposed.Introduction
Owing to the environmental and economic concerns in recent years, there has been a considerable upsurge of interest in the application of atom efficient catalytic methodologies to industrial organic synthesis. The synthesis of fine chemicals and complex organic molecules involving multisteps is normally associated with the production of large yields of by-products. As a result, the E-factor (weight of by-product per unit weight of product) is large and in the range 5–100.1 The development of alternative environmentally benign synthetic routes is thus of primary concern. In the context of developing processes with low E-factor and high atom efficiencies, the use of environmentally friendly heterogeneous catalysts assumes significance as it results in waste minimization, safe and simple operations and easy work-up. The use of solid acids such as clays and zeolites as ‘green’ catalysts has been well established.2,3 We have also exploited the catalytic potential of zeolites, mainly titanium silicate molecular sieve (TS-1), for various organic synthetic transformations.45-Hydroxy-2-(5H)-furanone is a key constituent in a number of biologically active compounds such as manoalide (a nonsteroidal anti-inflammatory agent), secomanolide, luffariellin, thoreotolide and cacospongiolide5etc. It has been used as a useful synthon in the total synthesis of portulal,6 (d,l)-pyrenophorin, (d,l)-strigol7 and camptothecin.8
Various methods for the synthesis of 5-hydroxy-2(5H)-furanone have been reported in the literature. The dye-sensitised oxygenation of furfural in ethanol followed by acid hydrolysis of the 5-ethoxy analogue is known to give 5-hydroxy-2(5H)-furanone.9 The hydroxy furanone and its derivatives have also been prepared by the oxidation of 2-furoic acid via singlet oxygen generated by photochemistry10 or by the reaction of furfural and hydrogen peroxide in the presence of a catalyst consisting of group V and VI metals of the periodic table.11 The photoinduced oxidation of furan/furfural in ethanol in the presence of eosin for 9–18 d is reported to give 5-hydroxy-2(5H)-furanone in reasonably good yield.12 However, many of these methods have several limitations: expensive and stoichiometric amount of the reagent, tedious work-up procedures, high temperature, multistep synthesis, low substrate concentration and long reaction times. Consequently there is a need to develop alternative reagents for this reaction. Here we report an efficient, high-yielding synthesis of 5-hydroxy-2(5H)-furanone by oxidation of furan over a TS-1/H2O2 system.
Experimental
Titanium silicate molecular sieve (TS-1) was prepared from tetraethylorthosilicate, tetrabutylorthotitanate, tetrapropylammonium hydroxide and water following the literature procedure.13 The Si∶Ti molar ratio of the sample was 33. The X-ray powder pattern of the calcinated sample of TS-1 is characteristic of the MFI structure. The Al-free titanium silicate (TS-1) retained its orthorhombic symmetry after calcination. A scanning electron micrograph of the TS-1 sample showed the absence of any amorphous material. The particle size of the cuboid-shaped crystallites range from 0.2 to 0.3 μm. The UV–VIS spectrum of TS-1 showed a band at 209 nm and the IR spectrum showed a characteristic absorption at 960 cm−1.In a typical experimental procedure, to a cooled solution (0 °C) of furan (2 g, 29.4 mmol) in acetonitrile (10 ml) were added TS-1 catalyst (0.4 g) and 25% H2O2 (9.6 g, 71 mmol). The mixture was stirred for 8 h and allowed to warm to room temperature. The catalyst was filtered off and the filtrate was concentrated and extracted with ethyl acetate, washed with aq. Na2SO3 and brine. The organic layer was separated and dried over anhydrous Na2SO4. Removal of solvent and subsequent silica gel column chromatography using light petroleum (bp 60–80 °C)∶ethyl acetate (3∶2) gave a colorless viscous oil which solidified on cooling. The solid was recrystallised from diethyl ether–light petroleum to afford 2.98 g (98.3%) of 5-hydroxy-2(5H)-furanone,14 mp 56–58 °C, (lit.,15 58–59 °C). The physical and spectroscopic data were in full agreement with the literature data.16,17
Results and discussion
When furan 1 was treated with aq. hydrogen peroxide in acetonitrile in the presence of TS-1, the corresponding hydroxy lactone 2 was obtained in excellent yield (Scheme 1). Thus, furan reacted smoothly with the TS-1/H2O2 system and this transformation is first of its kind in the literature. Under similar conditions, cyclic 1,3-dienes containing other heteroatoms, e.g. thiophene, mainly gave the sulfoxide and sulfone as major products and the hydroxy enones were not formed. The reaction with 1,3-acyclic and cyclic dienes failed due to the consecutive and multiple reaction pathways open to these dienes.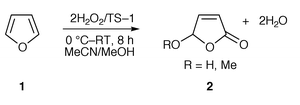 | ||
| Scheme 1 | ||
The influence of furan/H2O2 mole ratio on the conversion and product selectivity is presented in Table 1. With the increase in H2O2/furan ratio from 0.6 to 2.4, there was a significant increase in the product yield. Thus the reaction gave almost a quantitative yield of the desired hydroxy lactone 2 with the use of excess of hydrogen peroxide (ca. 2.4 equiv.).
| Substrate | ||||
|---|---|---|---|---|
| Entry | H2O2/Furan (mole ratio) | Furan | H2O2 (25%) | Product yielda |
| Reaction conditions: acetonitrile (10 ml), 0 °C–room temperature, 8 h, catalyst wt% with respect to furan = 20. a Yields refer to isolated pure product. | ||||
| 1 | 0.6 | 2 g (29.4 mmol) | 2.4 g (17.647 mmol) | 0.876 g (29.8%) |
| 2 | 1.2 | 2 g (29.4 mmol) | 4.8 g (35.29 mmol) | 1.74 g (59.2%) |
| 3 | 2.4 | 2 g (29.4 mmol) | 9.6 g (70.58 mmol) | 2.89 g (98.3%) |
Similarly, the effect of different solvents on furan conversion and product selectivity was studied. Aprotic solvents like acetone and acetonitrile seem to favor the formation of the hydroxy lactone vis-à-vis protic solvent like methanol. It was observed that when methanol was used as solvent, apart from the hydroxy lactone (25%), another major product, 5-methoxy-2(5H)-furanone (75% yield), characterised by spectroscopic data, was formed due to the subsequent methylation of the corresponding hydroxy compound.
A time dependent study of the oxidation of furan with H2O2 in the presence of a varying concentration of the TS-1 catalyst indicated that even a small amount of catalyst (7.5 wt%) can significantly catalyse and accelerate the rate of reaction. As the concentration of the catalyst was increased, the reaction became faster. Thus 20 wt% (on the basis of furan) catalyst was sufficient to oxidise furan completely affording the corresponding hydroxy lactone. The use of other catalysts such as Sn-silicalite-1, vanadium silicates such as VS-1 or VS-2, and Cr-silicalite-1 failed to accomplish the same transformation.
Regarding a possible mechanism, some of the reactive species, e.g. hydroxyperoxy, peroxy in the presence of H2O2, are routinely being proposed for oxidation processes involving TS-1.4,18,19 Alternatively, one could invoke the intermediacy of singlet oxygen20 in the oxidation of furan. Presumably, the generation of singlet oxygen could be visualised from the hydroperoxo or peroxo titanium species. Indeed, when the endoperoxide17 of furan was treated under TS-1 conditions, the formation of the hydroxy lactone 2 was observed indicating the intermediacy of 1O2 in TS-1/H2O2 chemistry (Scheme 2). It should be mentioned that the oxidation of furan by singlet oxygen generated photochemically17 or via the nonphotolytic method,21e.g. by the reaction of sodium hypochlorite and hydrogen peroxide, has also been widely investigated.
 | ||
| Scheme 2 | ||
Conclusion
In summary, a facile heterogeneous catalytic method for the oxidation of furan to the corresponding hydroxylactone has been developed. This method offers a practical alternative to conventional methods and the process itself is environmentally friendly with minimal waste.Acknowledgements
R.K.P. thanks the Director, NCL for allowing him to work as a guest worker. We thank Dr V. P. Shiralkar of the Catalysis Division for providing the TS-1 catalyst. NCL communication No. 6583.References
- R. A. Sheldon, Chem. Ind., 1997, 12 Search PubMed.
- J. M. Thomas and C. R. Theocharis, in Perspectives in Catalysis, ed. J. M. Thomas and K. I. Zamaraev, Blackwell Scientific Publications, London, 1992, p. 465. Search PubMed.
- C. B. Dartt and M. E. Davis, Catal. Today, 1994, 19, 151 CrossRef CAS.
- For an account of oxidative organic transformations catalysed by titanium and vanadium silicate molecular sieves, see P. Kumar, R. Kumar and B. Pandey, Synlett, 1995, 289 CrossRef CAS; R. S. Reddy, J. S. Reddy, R. Kumar and P. Kumar, J. Chem. Soc., Chem. Commun., 1992, 84 RSC; P. Kumar, V. R. Hegde, B. Pandey and T. Ravindranathan, J. Chem. Soc., Chem. Commun., 1993, 1553 RSC; A. Bhaumik, Godwin C. G. Pais, P. Kumar and R. Kumar, J. Catal., 1995, 156, 163 CrossRef; R. Kumar, Godwin C. G. Pais, B. Pandey and P. Kumar, J. Chem. Soc., Chem. Commun., 1995, 1315 RSC.
- E. Lattmann, J. Coombs and H. M. R. Hoffmann, Synthesis, 1996, 171 CrossRef CAS.
- A. Kanazawa, H. Kotsuki and T. Tokoroyama, Tetrahedron Lett., 1975, 3651 CrossRef.
- J. B. Heather, R. S. D. Mittal and C. J. Sih, J. Am. Chem. Soc., 1974, 96, 1976 CrossRef CAS.
- I. A. Meyers, R. L. Nolen, E. W. Collington, T. A. Norwid and R. C. Strickland, J. Org. Chem., 1974, 38, 1973.
- I. L. Doeer and R. E. Willette, J. Org. Chem., 1973, 38, 3878 CrossRef.
- J. A. Navio, J. F. Mota, M. A. Pradera Adrian and M. Garcia Gomez, J. Photochem. Photobiol. A, 1990, 52, 91 Search PubMed.
- V. V. Poskonin, L. A. Badovskaya, S. P. Gavrilova and V. G. Kul’nevi, Zh. Org. Khim., 1989, 25, 1701 Search PubMed.
- G. O. Schenck, Liebigs Ann. Chem., 1953, 584, 156 Search PubMed.
- A. Thangaraj, R. Kumar, S. P. Mirajkar and P. Ratnasamy, J. Catal., 1991, 130, 1 CrossRef CAS.
- The compound was fully characterised by physical and spectroscopic
data and also by comparison with an authentic sample. However, the
1H NMR of compound 2 (R
![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) H) showed the presence
of a minor amount of the hydrolysed product, fumaraldehydic acid..
H) showed the presence
of a minor amount of the hydrolysed product, fumaraldehydic acid.. - S. H. Schroeter, R. Appel, R. Brammer and G. O. Schenck, Justus Liebigs Ann. Chem., 1966, 697, 42 Search PubMed.
- M. D. Grove and D. Weisleder, J. Org. Chem., 1973, 38, 815 CrossRef CAS.
- G. M. C. Lee, E. T. Syage, D. A. Harcourt, J. M. Holmes and M. E. Gorst, J. Org. Chem., 1991, 56, 7007 CrossRef CAS.
- D. C. Huybrechts, L. D. Bruycker and P. A. Jacobs, Nature, 1990, 345, 240 CrossRef CAS.
- J. S. Reddy, U. R. Khire, P. Ratnasamy and R. B. Mitra, J. Chem. Soc., Chem. Commun., 1992, 1234 RSC.
- The singlet oxygen mediated ene reaction using zeolite as reaction media has recently been reported, see: V. Ramamurthy, Chem. Commun., 1998, 2411 RSC.
- C. S. Foote and S. Wexler, J. Am. Chem. Soc., 1964, 86, 3879 CrossRef CAS; C. S. Foote, S. Wexler, W. Ando and R. Higgins, J. Am. Chem. Soc., 1968, 90, 975 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2000 |
