Synthesis and reactivity of nitrido-rhenium and -osmium complexes with an oxygen tripod ligand
Received
(in Cambridge, UK)
16th August 1999
, Accepted 16th November 1999
First published on 24th December 1999
Abstract
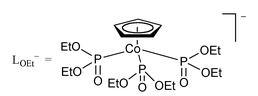
Interaction of [ReNCl3(PPh3)2] or [ReOCl2(PPh3)3] with NaLOEt (LOEt = [Co(η5-C5H5){PO(OEt)2}3]) afforded [ReLOEtN(PPh3)Cl] 1 and [ReLOEtOCl2] 2, respectively. Reaction of 1 with AgBF4 gave the nitridorhenium(VI) complex [ReLOEtN(PPh3)Cl]BF41·BF4, which has a μeff of 1.8 μB. Treatment of 1 with MeOSO2CF3, PhCH2Br or [Ph3C]BF4 afforded the respective organoimido species [ReLOEt(NMe)(PPh3)Cl][CF3SO3] 3, [ReLOEt(NCH2Ph)(PPh3)Cl]Br 4, and [ReLOEt(NCPh3)(PPh3)Cl] 5. Reaction of 1 with [Au(PPh3)(CF3SO3)], [Ru(Et2dtc)(PPh3)2(CO)(CF3SO3)], or [ReMeO3] yielded the bimetallic nitrido complexes [Au(PPh3){NReLOEt(PPh3)Cl}][CF3SO3] 6, [Ru(Et2dtc)(PPh3)(H2O)(CO){NReLOEt(PPh3)Cl}][CF3SO3] 7 or [ReMeO3{NReLOEt(PPh3)Cl}] 8, respectively. Treatment of [NBu4n][OsNCl4] with NaLOEt gave [OsLOEtNCl2] 9. The average Os–O, Os–Cl and Os–N distances in 9 are 2.066, 2.289 and 2.58(1) Å, respectively. Reaction of 9 with PPh3 afforded the osmium(IV) phosphoran iminate species [OsLOEt(NPPh3)Cl2] 10, which has a μeff of 2.0 μB. The average Os–O, Os–Cl and Os–N distances in 10 are 2.099, 2.342, 1.893(5) Å, respectively, the Os–N–P angle being 137.5(3)°. The formal potentials of the LOEt–Re and –Os complexes have been determined by cyclic voltammetry. On the basis of the ReVI–ReV formal potential, the π-donor strength was found to decrease in the order N3− > [NAu(PPh3)]2− > NMe2−.
Introduction
Transition metal nitrido complexes are of interest because of their applications in metal-mediated nitrogen atom transfer reactions.1–3 While early transition metal nitrides are basic and react with alkyl halides or Lewis acidic metal centres to give imido or μ-nitrido complexes, the late transition metal analogues exhibit electrophilic properties.4 In an effort to understand the factors governing reactivity of metal nitrides, isoelectronic nitrido complexes of ReV and OsVI were synthesized and their reactivities compared. Of particular interest are complexes of Re and Os with Kläui’s tripod ligand [Co(η5-C5H5){PO(OEt)2}3] or LOEt, which is known to stabilise metal ions in high oxidation states due to its π-donating capability.5 It may be noted that while Ru–LOEt complexes are well documented,5,6 there are few examples of the osmium congeners.7 In this paper we report on the synthesis and crystal structures of nitrido complexes of ReV and OsVI with LOEt and their reactivity toward electrophiles and PPh3.
Experimental
NMR Spectra were recorded on a Bruker ALX 300 spectrometer operating at 300 and 121.5 MHz for 1H and 31P, respectively. Chemical shifts (δ, ppm) are reported with reference to Si(CH3)4 (1H) and H3PO4 (31P). Infrared spectra (Nujol) were recorded on a Perkin-Elmer 16 PC FT-IR spectrophotometer. Cyclic voltammetry was performed with a Princeton Applied Research (PAR) Model 273A potentiostat. The working and reference electrodes were glassy carbon and Ag–AgNO3 (0.1 M in acetonitrile), respectively. Potentials were reported with reference to the ferrocenium–ferrocene couple (Cp2Fe+/0). Elemental analyses were performed by Medac Ltd, Surrey, UK.
Materials
The compounds [ReN(PPh3)2Cl2],8 [ReO(PPh3)2Cl3],9 [NBu4n][OsNCl4],10 [Au(PPh3)(CF3SO3)]![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 11 and [ReMeO3]
11 and [ReMeO3]![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 12 were prepared according to the literature methods. The triflate compound [Ru(Et2dtc)(PPh3)2(CO)(CF3SO3)] was synthesized by reaction of [Ru(Et2dtc)(PPh3)2(CO)H] (Et2dtc = N,N-diethyldithiocarbamate)
12 were prepared according to the literature methods. The triflate compound [Ru(Et2dtc)(PPh3)2(CO)(CF3SO3)] was synthesized by reaction of [Ru(Et2dtc)(PPh3)2(CO)H] (Et2dtc = N,N-diethyldithiocarbamate)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 13 with triflic acid (CF3SO3H) as described elsewhere.14 The compounds MeOSO2CF3, PhCH2Br and [Ph3C]BF4 were obtained from Aldrich and used as received.
13 with triflic acid (CF3SO3H) as described elsewhere.14 The compounds MeOSO2CF3, PhCH2Br and [Ph3C]BF4 were obtained from Aldrich and used as received.
Preparations
[ReLOEtN(PPh3)Cl]
1..
A mixture of [ReN(PPh3)2Cl2] (0.71 g, 0.90 mmol) and NaLOEt (0.5 g, 0.896 mmol) in tetrahydrofuran (thf![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )–toluene (50 cm3, 1∶1) was heated at reflux overnight. The volatiles were removed in vacuo and the residue was washed with hexane. Recrystallisation from Et2O–hexane afforded orange-red crystals, suitable for X-ray analysis (yield: 0.49 g, 54%). NMR (CDCl3): 1H, δ 0.77 (t, 3 H, CH3), 1.02 (t, 3 H, CH3), 1.18 (t, 3 H, CH3), 1.30 (t, 3 H, CH3), 1.36 (t, 3 H, CH3), 1.37 (t, 3 H, CH3), 2.71–2.93 (m, 2 H, OCH2), 3.54–3.69 (m, 2 H, OCH2), 3.97–4.05 (m, 2 H, OCH2), 4.29–4.04 (m, 6 H, OCH2), 4.99 (s, 5 H, C5H5) and 7.28–7.83 (m, 15 H, PPh3); 31P, δ 10.83 (s, PPh3) and 109.1–110.5 (m, P(O)(OEt)2). IR (cm−1): 946 [ν(Re
)–toluene (50 cm3, 1∶1) was heated at reflux overnight. The volatiles were removed in vacuo and the residue was washed with hexane. Recrystallisation from Et2O–hexane afforded orange-red crystals, suitable for X-ray analysis (yield: 0.49 g, 54%). NMR (CDCl3): 1H, δ 0.77 (t, 3 H, CH3), 1.02 (t, 3 H, CH3), 1.18 (t, 3 H, CH3), 1.30 (t, 3 H, CH3), 1.36 (t, 3 H, CH3), 1.37 (t, 3 H, CH3), 2.71–2.93 (m, 2 H, OCH2), 3.54–3.69 (m, 2 H, OCH2), 3.97–4.05 (m, 2 H, OCH2), 4.29–4.04 (m, 6 H, OCH2), 4.99 (s, 5 H, C5H5) and 7.28–7.83 (m, 15 H, PPh3); 31P, δ 10.83 (s, PPh3) and 109.1–110.5 (m, P(O)(OEt)2). IR (cm−1): 946 [ν(Re![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N)]. MS (FAB): m/z 1032 (M+) (Found: C, 40.2; H, 4.91; N, 1.32. Calc. for C35H50ClCoNO9P4Re: C, 40.7; H, 4.84; N, 1.36%).
N)]. MS (FAB): m/z 1032 (M+) (Found: C, 40.2; H, 4.91; N, 1.32. Calc. for C35H50ClCoNO9P4Re: C, 40.7; H, 4.84; N, 1.36%).
[ReLOEtOCl2]
2..
A mixture of [ReO(PPh3)2Cl3] (0.3 g, 0.361 mmol) and NaLOEt (0.15 g, 0.269 mmol) in thf–toluene (50 cm3, 1∶1) was heated at reflux overnight. The volatiles were removed in vacuo and the residue was washed with hexane. Recrystallisation from ether–hexane afforded yellow crystals (yield: 0.10 g, 48%). Despite several attempts, we have not been able to obtain good carbon analysis for complex 2. NMR (CDCl3): 1H, δ 1.26 (t, 6 H, CH3), 1.31 (t, 6 H, CH3), 1.33 (t, 6 H, CH3), 4.07–4.30 (m, 12 H, OCH2) and 5.14 (s, 5 H, C5H5); 31P-{1H}, δ 124.2 (m, P(O)(OEt)2). MS (FAB): m/z 808 (M+) (Found: C, 27.1; H, 4.32. Calc. for C17H35Cl2CoO10P3Re: C, 25.3; H, 4.34%).
[ReLOEtN(PPh3)Cl]BF41·BF4..
To a solution of complex 1 (80 mg, 0.078 mmol) in CH2Cl2 (25 cm3) was added AgBF4 (24 mg, 0.088 mmol). The resulting mixture was stirred for 40 min and filtered. The solvent was pumped off and the residue extracted with CH2Cl2. Recrystallisation from CH2Cl2–Et2O–hexane afforded brown crystals (109 mg, 53%). μeff = 1.7 μB (Found: C, 37.7; H, 4.48; N, 1.19. Calc. for C35H50BClCoF4NO9P4Re: C, 37.5; H, 4.47; N, 1.25%).
[ReLOEt(NMe)(PPh3)Cl][CF3SO3]
3..
To a solution of complex 1 (80 mg, 0.0784 mmol) in diethyl ether (20 cm3) was added MeOSO2CF3 (0.02 ml, 0.14 mmol) at 0 °C under nitrogen. The resulting mixture was allowed to warm to room temperature and stirred for 1 d during which it changed from red to yellow. The solvent was pumped off and the residue extracted with CH2Cl2. Recrystallisation from CH2Cl2–Et2O–hexane afforded yellow crystals (yield: 54 mg, 58%). NMR (CDCl3): 1H, δ 0.84 (t, 3 H, CH3), 1.08 (t, 3 H, CH3), 1.29–1.43 (t, overlapping, 12 H, CH3), 1.95 (d, 4JHP = 4.92 Hz, 3 H, NCH3), 3.14–3.26 (m, 2 H, OCH2), 3.52–3.58 (m, 2 H, OCH2), 4.09–4.36 (m, 8 H, OCH2), 5.11 (s, 5 H, C5H5) and 7.44–7.59 (m, 15 H, PPh3); 31P, δ
−11.38 (s, PPh3), 110.1, 119.5 and 124.0 (m, P(O)(OEt)2). MS (FAB): m/z 1047, (M − CF3SO3 + 1)+ (Found: C, 35.6; H, 4.34; N, 1.12. Calc. for C37H53ClCoF3N12P4ReS2·H2O: C, 36.0; H, 4.62; N, 1.14%).
[ReLOEt(NCH2Ph)(PPh3)Cl]Br 4..
This was prepared similarly as for complex 3 from 1 (80 mg, 0.0775 mmol) and PhCH2Br (0.02 cm3, 0.078 mmol) Recrystallisation from CH2Cl2–Et2O–hexane afforded yellow crystals (37 mg, 41%). NMR (CDCl3): 1H, δ 0.857 (t, 3 H, CH3), 1.06 (t, 3 H, CH3), 1.16 (t, 3 H, CH3), 1.22 (t, 3 H, CH3), 1.31 (t, 3 H, CH3), 1.34 (t, 3 H, CH3), 3.01–3.40 (m, 4 H, OCH2), 3.53–3.87 (m, 4 H, OCH2), 4.04–4.32 (m, 4 H, OCH2), 5.13 (s, 5 H, C5H5) and 6.91–7.58 (m, 20 H, phenyl protons); 31P-{1H}, δ
−12.6 (s, PPh3) and 124.1 (m, P(O)(OEt)2) (Found: C, 42.1; H, 4.98; N, 1.10. Calc. for C42H57BrClCoN9P4Re: C, 41.9; H, 4.74; N, 1.16%).
[ReLOEt(NCPh3)(PPh3)Cl]BF45..
This was prepared similarly as for complex 3 from 1 (94 mg, 0.091 mmol) and [CPh3]BF4 (30 mg, 0.09 mmol). Recrystallisation from CH2Cl2–Et2O–hexane afforded yellow crystals (74 mg, 60%). NMR (CDCl3): 1H, δ 0.80 (t, 3 H, CH3), 1.05 (t, 3 H, CH3), 1.18–1.29 (t, overlapping, 9 H, CH3), 1.34 (t, 3 H, CH3), 2.35–2.42 (m, 2 H, OCH2), 3.18–3.55 (m, 4 H, OCH2), 3.89–4.34 (m, 6 H, OCH2), 5.21 (s, 5 H, C5H5) and 6.92–7.33 (m, 30 H, phenyl protons). 31P-{1H}, δ
−10.64 (s, PPh3) and 119.9–124.5 (m, P(O)(OEt)2); 19F, δ
−155.0 (BF4). MS (FAB): m/z 1277, (M − BF4 + 2)+ (Found: C, 47.9; H, 4.86; N, 1.05. Calc. for C54H65BClCoF4NO9P4Re: C, 47.6; H, 4.77; N, 1.03%).
[Au(PPh3){NReLOEt(PPh3)Cl}][CF3SO3]
6..
This was prepared as for complex 3 from 1 (130 mg, 0.126 mmol) and [Au(PPh3)(CF3SO3)] (67 mg, 0.126 mmol) Recrystallisation from CH2Cl2–Et2O–hexane afforded greenish yellow crystals (109 mg, 53%). NMR (CDCl3): 1H, δ 0.82 (t, 3 H, CH3), 1.11 (t, 3 H, CH3), 1.21 (t, overlapping, 6 H, CH3), 1.29 (t, 3 H, CH3), 1.34 (t, 3 H, CH3), 2.90–3.11 (m, 2 H, OCH2), 3.44–3.63 (m, 6 H, OCH2), 4.05–4.39 (m, 4 H, OCH2), 5.05 (s, 5 H, C5H5) and 6.91–7.58 (m, 30 H, phenyl protons); 31P-{1H}, δ
−12.6 (s, RePPh3), 30.3 (s, AuPPh3) and 118.7–120.0 (m, P(O)(OEt)2). IR (cm−1): 953 [ν(Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) N–Au)]. MS (FAB): m/z 1492, (M − BF4 + 1)+ (Found: C, 39.2; H, 4.01; N, 0.84. Calc. for C54H65AuClCoF3NO12P5ReS: C, 39.5; H, 3.96; N, 0.85%).
N–Au)]. MS (FAB): m/z 1492, (M − BF4 + 1)+ (Found: C, 39.2; H, 4.01; N, 0.84. Calc. for C54H65AuClCoF3NO12P5ReS: C, 39.5; H, 3.96; N, 0.85%).
[Ru(Et2dtc)(PPh3)(H2O)(CO){NReLOEt(PPh3)Cl}][CF3SO3]
7..
This was prepared similarly as for complex 3 from 1 (100 mg, 0.097 mmol) and [Ru(Et2dtc)(PPh3)2(CO)(CF3SO3)] (92 mg, 0.097 mmol). Recrystallisation from CH2Cl2–hexane afforded brown crystals (yield: 82 mg, 49%). NMR (CDCl3): 1H, δ 0.77 (t, 6 H, CH3), 1.09 (t, 3 H, CH3), 1.18–1.30 (overlapping t, 9 H, CH3), 1.40 (t, 3 H, CH3), 2.61–3.04 (overlapping NCH2), 3.31–4.39 (m, 12 H, OCH2), 4.39 (m, 12 H, OCH2), 5.14 (s, 5 H, C5H5) and 7.10–7.48 (m, 30 H, phenyl protons); 31P-{1H}, δ 6.02 (s, RePPh3), 43.3 (s, RuPPh3) and 118.3–120.0 (m, PO(OEt)2). IR (cm−1): 1966 [ν(CO)]. MS (FAB): m/z 1572 (M+ − CF3SO3) (Found: C, 41.13; H, 4.45; N, 1.55. Calc. for C76H90ClCoF3N2O10P5ReRuS2: C, 41.01; H, 4.46; N, 1.62%).
[ReMeO3{NReLOEt(PPh3)Cl}]
8..
This was prepared similarly as for complex 3 from 1 (82 mg, 0.08 mmol) and [ReMeO3] (20 mg, 0.08 mmol). The red product was recrystallised from CH2Cl2–hexane (yield: 50 mg, 50%). The complex was found to be unstable and decomposed to 1 and ReMeO3 in solution, as reflected by NMR spectroscopy (see Results and discussion). Good analytical data were not obtained apparently because of dissociation of ReMeO3 during recrystallisation. IR (cm−1): 953 [ν(Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) N–Re)] and 920 [ν(Re
N–Re)] and 920 [ν(Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O)] (Found: C, 35.14; H, 4.55; N, 1.10. Calc. for C36H53ClCoNO9P4Re2: C, 33.71; H, 4.14; N, 1.09%).
O)] (Found: C, 35.14; H, 4.55; N, 1.10. Calc. for C36H53ClCoNO9P4Re2: C, 33.71; H, 4.14; N, 1.09%).
[OsLOEtNCl2]
9..
A mixture of [NBun4][OsNCl4] (60 mg, 0.098 mmol) and NaLOEt (50 mg, 0.0896 mmol) in acetone (40 cm3) was heated at reflux overnight. The volatiles were removed in vacuo and the residue was washed with hexane. Recrystallisation from CH2Cl2–hexane afforded deep red crystals, which are suitable for X-ray analysis (yield: 0.49 g, 54%). NMR (CDCl3): 1H, δ 1.21 (t, 6 H, CH3), 1.33 (t, 6 H, CH3), 1.35 (t, 6 H, CH3), 3.99–4.06 (m, 4 H, OCH2), 4.20–4.37 (m, 8 H, OCH2) and 5.10 (s, 5 H, C5H5); 31P-{1H}, δ 109.9, 122.6–123.7 (m, P(O)(OEt)2). MS (FAB): m/z 811, (M + 1)+ (Found: C, 25.4; H, 4.39; N, 1.73. Calc. for C17H35Cl2CoNO9OsP3: C, 25.2; H, 4.32; N, 1.73%).
[OsLOEt(NPPh3)Cl2]
10..
To a solution of complex 9 (50 mg, 0.062 mmol) in CH2Cl2 (25 cm3) was added PPh3 (20 mg, 0.076 mmol). The resulting mixture was stirred for 12 h, the solvent pumped off and the residue extracted with CH2Cl2. Recrystallisation from CH2Cl2–Et2O–hexane afforded brown crystals, suitable for X-ray analysis (yield: 32 mg, 49%). μeff = 2.0 μB (Found: C, 39.2; H, 4.73; N, 1.29. Calc. for C35H50Cl2CoN9OsP4: C, 39.2; H, 4.66; N, 1.31%).
X-Ray crystallography
A summary of pertinent crystallographic data and experimental details for complexes 1, 9 and 10 is shown in Table 1. All data were collected on a MAR research image-plate diffractometer using Mo-Kα radiation (λ = 0.71073 Å) with a graphite crystal monochromator in the incident beam. The diffracted intensities were corrected for Lorentz-polarisation effects. For 1 the nitride and chloride were found to be twofold disordered. A model with occupancies of 0.7 and 0.3 for the two sites was used for refinement to give a reasonable set of thermal and positional parameters. In complex 9 the phosphorus atoms of the tripod ligand LOEt exhibit twofold positional disorder. A model with occupancies of 0.5 each gave the best results in terms of both R factor and positional parameters of the ligand. All structures were solved by direct methods and refined on F by a full-matrix least-squares analysis. Non-hydrogen atoms, except the disordered nitride and chloride of complex 1, were refined anisotropically. Calculations were performed on a Silicon-Graphics computer, using the program package TEXSAN.15 Hydrogen atoms were included and fixed in their idealised positions (C–H 0.95 Å). Selected bond lengths and angles for 1, 9 and 10 are listed in Tables 2–4, respectively.
Table 1 Crystallographic data and experimental details for [ReLOEtN(PPh3)Cl] 1, [OsLOEtNCl2] 9 and [OsLOEt(NPPh3)Cl2] 10
| |
1
|
9
|
10
|
| Empirical formula |
C35H50ClCoNO9P4Re |
C17H35Cl2CoNO9OsP3 |
C35H50Cl2CoNO9OsP4 |
|
M
|
1033.27 |
810.43 |
1072.72 |
| Crystal system |
Monoclinic |
Monoclinic |
Monoclinic |
| Space group |
P![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 21/n (no. 14) 21/n (no. 14) |
P![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 21/n (no. 14) 21/n (no. 14) |
P![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 21/c (no. 14) 21/c (no. 14) |
|
a/Å |
14.031(1) |
12.326(1) |
18.414(2) |
|
b/Å |
14.582(1) |
18.623(1) |
12.214(3) |
|
c/Å |
20.993(2) |
12.820(1) |
19.449(1) |
|
β/° |
92.58(1) |
94.49(2) |
106.40(9) |
|
V/Å3 |
4290.8(4) |
2933.8(4) |
4196.3(6) |
|
Z
|
4 |
4 |
4 |
|
D
calc/g cm−3 |
1.599 |
1.835 |
1.513 |
|
T/K |
298 |
298 |
298 |
|
μ/mm−1 |
3.467 |
5.277 |
3.749 |
| No. reflections measured |
8244 |
5513 |
8165 |
| No. reflections observed |
5224 |
4088 |
6731 |
R(F![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) ) |
0.042 |
0.056 |
0.035 |
R![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′ ′ |
0.045 |
0.069 |
0.062 |
Table 2 Selected bond lengths (Å) and angles (°) for [ReLOEtN(PPh3)Cl] 1
| Re(1)–Cl(1) |
2.292(3) |
Re(1)–Cl(2) |
2.216(6) |
| Re(1)–P(4) |
2.372(1) |
Re(1)–O(1) |
2.251(3) |
| Re(1)–O(2) |
2.179(4) |
Re(1)–O(3) |
2.115(3) |
| Re(1)–N(1) |
1.795(8) |
Re(1)–N(2) |
1.97(2) |
| |
|
|
|
| C(1)–Re(1)–Cl(2) |
102.3(2) |
Cl(1)–Re(1)–P(4) |
89.54(8) |
| Cl(1)–Re(1)–O(1) |
88.5(1) |
Cl(1)–Re(1)–O(2) |
165.5(1) |
| Cl(1)–Re(1)–O(3) |
89.7(1) |
Cl(1)–Re(1)–N(1) |
101.8(2) |
| Cl(1)–Re(1)–N(2) |
8.9(7) |
Cl(2)–Re(1)–P(4) |
99.4(2) |
| Cl(2)–Re(1)–O(1) |
164.7(2) |
Cl(2)–Re(1)–O(2) |
90.8(2) |
| Cl(2)–Re(1)–O(3) |
87.8(2) |
Cl(2)–Re(1)–N(1) |
12.6(3) |
| Cl(2)–Re(1)–N(2) |
93.6(7) |
P(4)–Re(1)–O(1) |
91.32(9) |
| P(4)–Re(1)–O(2) |
94.6(1) |
P(4)–Re(1)–O(3) |
172.7(1) |
| P(4)–Re(1)–N(1) |
86.9(3) |
P(4)–Re(1)–N(2) |
89.3(7) |
| O(1)–Re(1)–O(2) |
94.6(1) |
O(1)–Re(1)–O(3) |
81.4(1) |
| O(1)–Re(1)–N(1) |
169.5(2) |
O(1)–Re(1)–N(2) |
97.4(7) |
| O(2)–Re(1)–O(3) |
84.4(1) |
O(2)–Re(1)–N(1) |
92.3(2) |
| O(2)–Re(1)–N(2) |
173.6(7) |
O(3)–Re(1)–N(1) |
100.4(3) |
| O(3)–Re(1)–N(2) |
91.1(7) |
N(1)–Re(1)–N(2) |
92.9(7) |
Table 3 Selected bond lengths (Å) and angles (°) for [OsLOEtNCl2] 9
| Os(1)–Cl(1) |
2.300(4) |
Os(1)–Cl(2) |
2.277(7) |
| Os(1)–O(1) |
2.022(8) |
Os(1)–O(2) |
2.159(8) |
| Os(1)–O(3) |
2.017(8) |
Os(1)–N(1) |
1.58(1) |
| |
|
|
|
| Cl(1)–Os(1)–Cl(4) |
87.7(2) |
Cl(1)–Os(1)–O(1) |
167.7(4) |
| Cl(1)–Os(1)–O(2) |
87.6(3) |
Cl(1)–Os(1)–O(3) |
89.1(3) |
| Cl(1)–Os(1)–N(1) |
96.8(4) |
Cl(2)–Os(1)–O(1) |
90.4(3) |
| Cl(2)–Os(1)–O(2) |
86.5(4) |
Cl(2)–Os(1)–O(3) |
166.0(4) |
| Cl(2)–Os(1)–N(1) |
98.0(5) |
O(1)–Os(1)–O(2) |
80.1(5) |
| O(1)–Os(1)–O(3) |
89.8(3) |
O(1)–Os(1)–N(1) |
95.6(6) |
| O(2)–Os(1)–O(3) |
79.7(4) |
O(2)–Os(1)–N(1) |
173.8(4) |
| O(3)–Os(1)–N(1) |
96.0(5) |
|
Table 4 Selected bond lengths (Å) and angles (°) for [OsLOEt(NPPh3)Cl2] 10
| Os(1)–Cl(1) |
2.338(2) |
Os(1)–Cl(2) |
2.346(2) |
| Os(1)–O(1) |
2.110(4) |
Os(1)–O(2) |
2.097(4) |
| Os(1)–O(3) |
2.090(4) |
Os(1)–N(1) |
1.893(5) |
| P(4)–N(1) |
1.575(5) |
|
|
| |
|
|
|
| Cl(1)–Os(1)–Cl(2) |
91.63(7) |
Cl(1)–Os(1)–O(1) |
87.7(1) |
| Cl(1)–Os(1)–O(2) |
174.1(1) |
Cl(1)–Os(1)–O(3) |
91.9(1) |
| Cl(1)–Os(1)–N(1) |
94.7(2) |
Cl(2)–Os(1)–O(1) |
89.7(1) |
| Cl(2)–Os(1)–O(2) |
89.1(1) |
Cl(2)–Os(1)–O(3) |
173.8(1) |
| Cl(2)–Os(1)–N(1) |
94.3(2) |
O(1)–Os(1)–O(2) |
86.4(2) |
| O(1)–Os(1)–O(3) |
85.4(2) |
O(1)–Os(1)–N(1) |
175.2(2) |
| O(2)–Os(1)–O(3) |
86.8(2) |
O(2)–Os(1)–N(1) |
91.1(2) |
| O(3)–Os(1)–N(1) |
90.4(2) |
Os(1)–N(1)–P(4) |
137.5(3) |
CCDC reference number 186/1741.
Results and discussion
LOEtRe complexes
The syntheses of LOEtRe complexes are summarised in Scheme 1. Interaction of [ReNCl2(PPh3)2] and [ReOCl3(PPh3)2] with NaLOEt afforded air-stable [ReLOEtN(PPh3)Cl] 1 and [ReLOEtOCl2] 2, respectively. The IR spectrum of 1 shows a peak at 946 cm−1, which is tentatively assigned as ν(Re![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N). The ν(Re
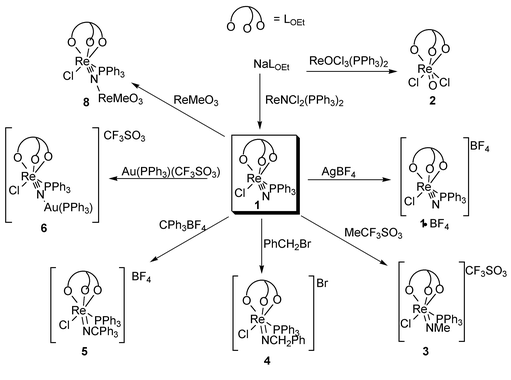
N). The ν(Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) for 2 was not assigned due to the presence of ligand bands in the region. X-Ray quality crystals of complex 1 were obtained by recrystallisation from CH2Cl2–hexane. However, the chloride and nitride in 1 were found to be twofold disordered. A model with site occupancies of 0.3 and 0.7 for the two sites was used for the refinement. Fig. 1 shows a perspective view of the molecule; selected bond lengths and angles are listed in Table 2. The Re–P and average Re–O distances were found to be 2.372(1) and 2.183 Å. Oxidation of 1 with AgBF4 afforded [ReLOEtN(PPh3)Cl]BF41·BF4. The measured μeff of 1.8 μB is consistent with the formulation of ReVI. Although the ReVI–ReV couple for 2 is reversible (see later section), no well defined products were obtained for oxidation of 2 with silver(I) salts. The nitride in 1 was found to be nucleophilic and react with electrophiles to give imidorhenium(V) complexes. Thus, treatment of 1 with MeOSO2CF3 afforded the methylimido complex [ReLOEt(NMe)(PPh3)Cl][CF3SO3] 3. The N-methyl protons in 3 were found to couple with the phosphorus of PPh3 and appear as a doublet at δ 1.95 (4JHP = 4.92 Hz). Similarly interaction of 1 with PhCH2Br or [Ph3C]BF4 gave the respective imido species [ReLOEt(NCH2Ph)(PPh3)Cl]Br 4 or [ReLOEt(NCPh3)(PPh3)Cl]BF45. These imidorhenium(V) complexes are air stable in both the solid state and solution.
O) for 2 was not assigned due to the presence of ligand bands in the region. X-Ray quality crystals of complex 1 were obtained by recrystallisation from CH2Cl2–hexane. However, the chloride and nitride in 1 were found to be twofold disordered. A model with site occupancies of 0.3 and 0.7 for the two sites was used for the refinement. Fig. 1 shows a perspective view of the molecule; selected bond lengths and angles are listed in Table 2. The Re–P and average Re–O distances were found to be 2.372(1) and 2.183 Å. Oxidation of 1 with AgBF4 afforded [ReLOEtN(PPh3)Cl]BF41·BF4. The measured μeff of 1.8 μB is consistent with the formulation of ReVI. Although the ReVI–ReV couple for 2 is reversible (see later section), no well defined products were obtained for oxidation of 2 with silver(I) salts. The nitride in 1 was found to be nucleophilic and react with electrophiles to give imidorhenium(V) complexes. Thus, treatment of 1 with MeOSO2CF3 afforded the methylimido complex [ReLOEt(NMe)(PPh3)Cl][CF3SO3] 3. The N-methyl protons in 3 were found to couple with the phosphorus of PPh3 and appear as a doublet at δ 1.95 (4JHP = 4.92 Hz). Similarly interaction of 1 with PhCH2Br or [Ph3C]BF4 gave the respective imido species [ReLOEt(NCH2Ph)(PPh3)Cl]Br 4 or [ReLOEt(NCPh3)(PPh3)Cl]BF45. These imidorhenium(V) complexes are air stable in both the solid state and solution.
 |
| | Scheme 1 | |
![Perspective view of [ReLOEtN(PPh3)Cl] 1.](/image/article/2000/DT/a906618g/a906618g-f1.gif) |
| | Fig. 1 Perspective view of [ReLOEtN(PPh3)Cl] 1.
| |
Bimetallic nitrido complexes containing LOEtRe
Reaction of complex 1 with organometallic triflates afforded bimetallic nitrido complexes. Thus, 1 reacted with [Au(PPh3)(CF3SO3)] to give [Au(PPh3){NReLOEt(PPh3)Cl}][CF3SO3] 6, isolated as an air-stable yellow solid. No reactions were found between 1 and [Ir(CO)(PPh3)2(CF3SO3)], which was prepared in situ from trans-[Ir(CO)(PPh3)2Cl] and Ag(CF3SO3), possibly because of steric reasons. The IR spectrum of 6 shows ν(Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) N–Au) at 953 cm−1, which is higher than the ν(Re
N–Au) at 953 cm−1, which is higher than the ν(Re![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) for 1. Enhancement of the metal–nitride stretching frequency upon formation of μ-nitride bridges has also been observed for binuclear complexes of Os nitrides, e.g. in [Au(PPh3)(NOsO3)].16 Reaction of 1 with [Ru(Et2dtc)(PPh3)2(CO)(CF3SO3)],14 which was prepared from [Ru(Et2dtc)(PPh3)2(CO)H]
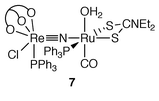
N) for 1. Enhancement of the metal–nitride stretching frequency upon formation of μ-nitride bridges has also been observed for binuclear complexes of Os nitrides, e.g. in [Au(PPh3)(NOsO3)].16 Reaction of 1 with [Ru(Et2dtc)(PPh3)2(CO)(CF3SO3)],14 which was prepared from [Ru(Et2dtc)(PPh3)2(CO)H]![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 13 and triflic acid, afforded [Ru(Et2dtc)(PPh3)(H2O)(CO){NReLOEt(PPh3)Cl}][CF3SO3] 7. One PPh3 dissociated from Ru upon formation of 7 possibly due to steric congestion in the bimetallic complex. A preliminary diffraction study of 7 showed that the aqua ligand, presumably derived from moisture in the solvent, is trans to the CO, and the nitride is trans to a sulfur of Et2dtc as shown below.
13 and triflic acid, afforded [Ru(Et2dtc)(PPh3)(H2O)(CO){NReLOEt(PPh3)Cl}][CF3SO3] 7. One PPh3 dissociated from Ru upon formation of 7 possibly due to steric congestion in the bimetallic complex. A preliminary diffraction study of 7 showed that the aqua ligand, presumably derived from moisture in the solvent, is trans to the CO, and the nitride is trans to a sulfur of Et2dtc as shown below.
Reaction of 1 with [ReMeO3] afforded the adduct [ReMeO3{NReLOEt(PPh3)Cl}] 8. While complex 8 is stable in the solid state, it decomposed readily to 1 and [ReMeO3] in solution. The NMR spectrum of 8 in CDCl3 only shows respective signals due to 1 and [ReMeO3]. The IR Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O stretching frequency for 8 of 920 cm−1 is lower than that for [ReMeO3] (950 cm−1),17 indicating that the Re
O stretching frequency for 8 of 920 cm−1 is lower than that for [ReMeO3] (950 cm−1),17 indicating that the Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O bonds are weakened upon adduct formation. Like 6, the Re
O bonds are weakened upon adduct formation. Like 6, the Re![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching frequency for 8 (953 cm−1) is higher than that for 1.
N stretching frequency for 8 (953 cm−1) is higher than that for 1.
LOEtOs complexes
Interaction of [NBun4][OsNCl4] with NaLOEt in boiling acetone afforded [OsLOEtNCl2] 9, isolated as air-stable deep red crystals. No reactions were found between [PPh4]2[OsO2Cl4]![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 18 and NaLOEt possibly because of the preference of OsVI for trans-O
18 and NaLOEt possibly because of the preference of OsVI for trans-O![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) Os
Os![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O geometry. The crystal structure of 9 has been determined and is shown in Fig. 2. Selected bond lengths and angles are listed in Table 3. The geometry around Os is essentially octahedral. The Os–N and average Os–Cl in 9 are 1.58(1) and 2.289 Å, respectively. The Os–O bond that is trans to the nitride (2.159(8) Å) is significantly longer than the other two Os–O bonds (average 2.039 Å), indicative of trans influence of the nitride. The IR Os–N stretching frequency was not assigned due to the presence of ligand bands in the 1000–1100 cm−1 region.
O geometry. The crystal structure of 9 has been determined and is shown in Fig. 2. Selected bond lengths and angles are listed in Table 3. The geometry around Os is essentially octahedral. The Os–N and average Os–Cl in 9 are 1.58(1) and 2.289 Å, respectively. The Os–O bond that is trans to the nitride (2.159(8) Å) is significantly longer than the other two Os–O bonds (average 2.039 Å), indicative of trans influence of the nitride. The IR Os–N stretching frequency was not assigned due to the presence of ligand bands in the 1000–1100 cm−1 region.
![Perspective view of [OsLOEtNCl2] 9.](/image/article/2000/DT/a906618g/a906618g-f2.gif) |
| | Fig. 2 Perspective view of [OsLOEtNCl2] 9.
| |
No alkylation occurred when complex 9 was treated with MeOSO2CF3, PhCH2Br or [Ph3C]BF4, indicating that the osmium nitrido complex is less nucleophilic than the rhenium analogue 1. In contrast to electrophilic trans-[OsN(tpy)Cl2]+ (tpy = 2,2′∶6′,2′′-terpyridine)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 19 and [OsTpNCl2] (Tp = hydridotris(pyrazol-1-yl)borate),20 complex 9 does not react with nucleophiles such as NaN3, Me3NO and propylene sulfide. Reaction of 9 with PPh3 in CH2Cl2 afforded the osmium(IV) phosphoraniminato complex [OsLOEt(NPPh3)Cl2] 10, which was characterised by X-ray diffraction. Fig. 3 shows a perspective view of 10, selected bond lengths and angles are given in Table 4. The Os–N distance in 10 of 1.893(5) Å is comparable to that in trans-[Os(tpy)Cl2(NPPh3)]+ (2.093(5) Å),21 consistent with the formulation of a Os–N single bond. The P–N bond distance of 1.575(5) Å is similar to that in trans-[Os(tpy)Cl2(NPPh3)]+ (1.618(5) Å)
19 and [OsTpNCl2] (Tp = hydridotris(pyrazol-1-yl)borate),20 complex 9 does not react with nucleophiles such as NaN3, Me3NO and propylene sulfide. Reaction of 9 with PPh3 in CH2Cl2 afforded the osmium(IV) phosphoraniminato complex [OsLOEt(NPPh3)Cl2] 10, which was characterised by X-ray diffraction. Fig. 3 shows a perspective view of 10, selected bond lengths and angles are given in Table 4. The Os–N distance in 10 of 1.893(5) Å is comparable to that in trans-[Os(tpy)Cl2(NPPh3)]+ (2.093(5) Å),21 consistent with the formulation of a Os–N single bond. The P–N bond distance of 1.575(5) Å is similar to that in trans-[Os(tpy)Cl2(NPPh3)]+ (1.618(5) Å)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 21 and is typical for a P
21 and is typical for a P![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) N double bond. As expected, the average Os–Cl distance in 10 (2.342 Å) is longer than that in 9. Unlike most osmium(IV) phosphoran iminato complexes that contain linear Os–N
N double bond. As expected, the average Os–Cl distance in 10 (2.342 Å) is longer than that in 9. Unlike most osmium(IV) phosphoran iminato complexes that contain linear Os–N![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) PR3 moieties,22 the Os–N–P linkage in 10 is bent with an angle of 137.5(3)°. The Os–N–P angle is slightly bigger than that in trans-[Os(tpy)Cl2(NPPh3)]+ (132.5(3)°).21 The measured magnetic moment for 10 of ca. 2.0 μB is different from the spin–only value for two unpaired electrons (2.83 μB) because of spin–orbit coupling.21
PR3 moieties,22 the Os–N–P linkage in 10 is bent with an angle of 137.5(3)°. The Os–N–P angle is slightly bigger than that in trans-[Os(tpy)Cl2(NPPh3)]+ (132.5(3)°).21 The measured magnetic moment for 10 of ca. 2.0 μB is different from the spin–only value for two unpaired electrons (2.83 μB) because of spin–orbit coupling.21
![Perspective view of [OsLOEt(NPPh3)Cl2] 10.](/image/article/2000/DT/a906618g/a906618g-f3.gif) |
| | Fig. 3 Perspective view of [OsLOEt(NPPh3)Cl2] 10.
| |
Electrochemistry
The formal potentials of the LOEt–Re and –Os complexes have been determined by cyclic voltammetry and are summarised in Table 5. The cyclic voltammogram (CV) of 1 exhibits a reversible couple at −0.296 V vs. Cp2Fe+/0, which is assigned as the ReVI–ReV couple. Consistent with the assignment, the isolated rhenium(VI) complex 1·BF4 was reduced at the same potential. The ReVI–ReV potential for 2 (0.470 V) is more anodic than that for 1, consistent with the higher π-donor strength of nitride compared with oxide. Oxidation of cationic imido complexes 3–5 is irreversible. The irreversible waves at 0.620, 0.705 and 0.557 V for complexes 3–5 are tentatively assigned as the respective ReVI–ReV oxidation. The dimetallic nitrido complexes 6 and 7 exhibit reversible ReVI–ReV couples at 0.161 and 0.08 V, respectively, which are higher than that for 1 but lower than those for 3–5. On the basis of the ReVI–ReV potential for [ReLOEt(X)(PPh3)Cl], the π-donor strength of X is ranked in the order N3− > [N{Au(PPh3)}]2− > NR2−. This trend is consistent with that found for [Os(X)O3] [X = N, NBut or NAu(PPh3)].16 Complex 10 exhibits a reversible reduction at −0.718 V, which is tentatively assigned as the OsIV–OsIII couple. The OsIV–OsIII potential for 10 is less anodic than that for [Os(tpy)Cl2(NPPh3)]+ (−0.27 V vs. standard calomel electrode),21 indicative of the ability of the LOEt in stabilising Os in high oxidation states.
Table 5 Formal potentials (E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) °) for Re– and Os–LOEt complexes
°) for Re– and Os–LOEt complexes
| Complex |
E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ° °![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a/V vs. Cp2Fe+/0 a/V vs. Cp2Fe+/0 |
|
Potential measured in CH2Cl2 with 0.1 mol dm−3 [NBun4]PF6 as supporting electrolyte; scan rate = 100 mV s−1.
Irreversible.
|
| [ReLOEtN(PPh3)Cl] |
−0.296 |
| [ReLOEtOCl2] |
0.470 |
| [ReLOEt(NMe)(PPh3)Cl]+ |
0.620![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
| [ReLOEt(NCPh3)(PPh3)Cl]+ |
0.705![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
| [ReLOEt(NCH2Ph)(PPh3)Cl]+ |
0.557![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
| [Au(PPh3){NReLOEt(PPh3)Cl}]+ |
0.161 |
| [Ru(Et2dtc)(PPh3)(H2O)(CO){NReLOEt(PPh3)Cl}]+ |
0.080 |
| [OsLOEt(NPPh3)Cl2] |
−0.718 |
In summary, we have isolated the first nitrido complexes of ReV and OsVI with LOEt. While the rhenium complex is nucleophilic and reacts with electrophiles to give imido or μ-nitrido complexes, the osmium analogue reacts with PPh3 to give a phosphoraminatoosmium(IV) complex. On the basis of cyclic voltammetry, the π-donor strength for multiply bonded ligands is ranked in the order: N3− > (NMLn)2− > NR2− ≈ O2−.
Acknowledgements
The financial support from the Hong Kong Research Grants Council (project no. HKUST6066/98P) is gratefully acknowledged.
References
- J. T. Groves and T. Takahashi, J. Am. Chem. Soc., 1983, 105, 2073 CrossRef CAS; J. Du Bois, C. S. Tomooka, J. Hong and E. M. Carreira, Acc. Chem. Res., 1997, 30, 364 CrossRef CAS and refs. therein..
- L. A. Bottomley and F. L. Neely, J. Am. Chem. Soc., 1989, 111, 5955 CrossRef CAS; C. Tong and L. A. Bottomley, Inorg. Chem., 1996, 35, 5108 CrossRef CAS; L. K. Woo, Chem. Rev., 1993, 93, 1125 CrossRef CAS and refs. therein. C. J. Chang, D. W. Low and H. B. Gray, Inorg. Chem., 1997, 36, 270 CrossRef CAS.
- C. E. Laplaza, A. R. Johnson and C. C. Cummins, J. Am. Chem. Soc., 1996, 118, 709 CrossRef CAS.
-
W. A. Nugent
and J. M. Mayer
, Metal–Ligand Multiple Bonds, Wiley, New York, 1998;
Search PubMed; K. Dehnicke and J. Strähle, Angew. Chem., Int. Ed. Engl., 1992, 31, 955 Search PubMed.
- W. Kläui, Angew. Chem., Int. Ed. Engl., 1990, 29, 627 CrossRef.
- J. M. Power, K. Evertz, L. Henling, R. Marsch, W. P. Schaefer, J. A. Labinger and J. E. Bercaw, Inorg. Chem., 1990, 29, 5058 CrossRef CAS; U. Kölle, G. Flunkert, R. Borissen, M. U. Schmidt and U. Englert, Angew. Chem., Int. Ed. Engl., 1992, 113, 3611; W.-H. Leung, E. Y. Y. Chan and W.-T. Wong, Organometallics, 1997, 16, 3234 CrossRef CAS.
- A. M. LaPointe and R. R. Schrock, Organometallics, 1993, 12, 3379 CrossRef CAS.
- B. P. Sullivan, J. C. Brewer, H. B. Gray, D. Linebarrier and J. M. Mayer, Inorg. Synth., 1992, 29, 146 CAS.
- G. Parshall, Inorg. Synth., 1977, 17, 100.
- W. P. Griffith and D. Paulson, J. Chem. Soc., Dalton Trans., 1973, 1315 RSC.
- P. B. Braunstein, H. Lehner, D. Matt, K. Burgess and M. J. Ohlmeyer, Inorg. Synth., 1990, 27, 218 CAS.
- W. A. Herrmann, F. E. Kühn, W. Fischer, W. R. Thiel and C. C. Romao, Inorg. Chem., 1992, 31, 4431 CrossRef CAS.
- P. B. Critchow and S. D. Robinson, J. Chem. Soc., Dalton Trans., 1975, 1367 RSC.
-
W.-H. Leung
, H. G. Zheng
, J. L. C. Chim
, T. Wong
, W. Lai
, C.-H. Lam
, I. D. Williams
and W.-T. Wong
, submitted for publication.
.
-
TEXSAN Crystal Structure Analysis Package, Molecular Structure Corporation, Houston, TX, 1985 and 1992.
Search PubMed.
- W.-H. Leung, J. L. C. Chim and W.-T. Wong, J. Chem. Soc., Dalton Trans., 1997, 3277 RSC.
- W. A. Herrmann, J. G. Kuchler, G. Weichselbaumer, E. Herdtweck and P. Kiprof, J. Organomet. Chem., 1989, 272, 351 CrossRef CAS.
- P. A. Shapley, H. S. Kim and S. R. Wilson, Organometallics, 1988, 7, 928 CrossRef CAS.
- K. D. Demadis, E.-S. El-Samanody, T. J. Meyer and P. S. White, Inorg. Chem., 1998, 37, 838 CrossRef CAS; K. D. Demadis, T. J. Meyer and P. S. White, Inorg. Chem., 1998, 37, 3610 CrossRef CAS.
- T. C. Crevier, S. Lovell, J. M. Mayer, A. L. Rheingold and I. A. Guzei, J. Am. Chem. Soc., 1998, 120, 6607 CrossRef CAS.
- M. Bakir, P. S. White, A. Dovletoglou and T. J. Meyer, Inorg. Chem., 1991, 30, 2835 CrossRef CAS.
- J. Strähle and K. Dehnicke, Polyhedron, 1989, 8, 707 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2000 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 11 and [ReMeO3]
11 and [ReMeO3]![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 12 were prepared according to the literature methods. The triflate compound [Ru(Et2dtc)(PPh3)2(CO)(CF3SO3)] was synthesized by reaction of [Ru(Et2dtc)(PPh3)2(CO)H] (Et2dtc = N,N-diethyldithiocarbamate)
12 were prepared according to the literature methods. The triflate compound [Ru(Et2dtc)(PPh3)2(CO)(CF3SO3)] was synthesized by reaction of [Ru(Et2dtc)(PPh3)2(CO)H] (Et2dtc = N,N-diethyldithiocarbamate)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 13 with triflic acid (CF3SO3H) as described elsewhere.14 The compounds MeOSO2CF3, PhCH2Br and [Ph3C]BF4 were obtained from Aldrich and used as received.
13 with triflic acid (CF3SO3H) as described elsewhere.14 The compounds MeOSO2CF3, PhCH2Br and [Ph3C]BF4 were obtained from Aldrich and used as received.
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )–toluene (50 cm3, 1∶1) was heated at reflux overnight. The volatiles were removed in vacuo and the residue was washed with hexane. Recrystallisation from Et2O–hexane afforded orange-red crystals, suitable for X-ray analysis (yield: 0.49 g, 54%). NMR (CDCl3): 1H, δ 0.77 (t, 3 H, CH3), 1.02 (t, 3 H, CH3), 1.18 (t, 3 H, CH3), 1.30 (t, 3 H, CH3), 1.36 (t, 3 H, CH3), 1.37 (t, 3 H, CH3), 2.71–2.93 (m, 2 H, OCH2), 3.54–3.69 (m, 2 H, OCH2), 3.97–4.05 (m, 2 H, OCH2), 4.29–4.04 (m, 6 H, OCH2), 4.99 (s, 5 H, C5H5) and 7.28–7.83 (m, 15 H, PPh3); 31P, δ 10.83 (s, PPh3) and 109.1–110.5 (m, P(O)(OEt)2). IR (cm−1): 946 [ν(Re
)–toluene (50 cm3, 1∶1) was heated at reflux overnight. The volatiles were removed in vacuo and the residue was washed with hexane. Recrystallisation from Et2O–hexane afforded orange-red crystals, suitable for X-ray analysis (yield: 0.49 g, 54%). NMR (CDCl3): 1H, δ 0.77 (t, 3 H, CH3), 1.02 (t, 3 H, CH3), 1.18 (t, 3 H, CH3), 1.30 (t, 3 H, CH3), 1.36 (t, 3 H, CH3), 1.37 (t, 3 H, CH3), 2.71–2.93 (m, 2 H, OCH2), 3.54–3.69 (m, 2 H, OCH2), 3.97–4.05 (m, 2 H, OCH2), 4.29–4.04 (m, 6 H, OCH2), 4.99 (s, 5 H, C5H5) and 7.28–7.83 (m, 15 H, PPh3); 31P, δ 10.83 (s, PPh3) and 109.1–110.5 (m, P(O)(OEt)2). IR (cm−1): 946 [ν(Re![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N)]. MS (FAB): m/z 1032 (M+) (Found: C, 40.2; H, 4.91; N, 1.32. Calc. for C35H50ClCoNO9P4Re: C, 40.7; H, 4.84; N, 1.36%).
N)]. MS (FAB): m/z 1032 (M+) (Found: C, 40.2; H, 4.91; N, 1.32. Calc. for C35H50ClCoNO9P4Re: C, 40.7; H, 4.84; N, 1.36%).
![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) N–Au)]. MS (FAB): m/z 1492, (M − BF4 + 1)+ (Found: C, 39.2; H, 4.01; N, 0.84. Calc. for C54H65AuClCoF3NO12P5ReS: C, 39.5; H, 3.96; N, 0.85%).
N–Au)]. MS (FAB): m/z 1492, (M − BF4 + 1)+ (Found: C, 39.2; H, 4.01; N, 0.84. Calc. for C54H65AuClCoF3NO12P5ReS: C, 39.5; H, 3.96; N, 0.85%).
![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) N–Re)] and 920 [ν(Re
N–Re)] and 920 [ν(Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O)] (Found: C, 35.14; H, 4.55; N, 1.10. Calc. for C36H53ClCoNO9P4Re2: C, 33.71; H, 4.14; N, 1.09%).
O)] (Found: C, 35.14; H, 4.55; N, 1.10. Calc. for C36H53ClCoNO9P4Re2: C, 33.71; H, 4.14; N, 1.09%).
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 21/n (no. 14)
21/n (no. 14)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 21/n (no. 14)
21/n (no. 14)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 21/c (no. 14)
21/c (no. 14)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )
)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′
′![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N). The ν(Re
N). The ν(Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) for 2 was not assigned due to the presence of ligand bands in the region. X-Ray quality crystals of complex 1 were obtained by recrystallisation from CH2Cl2–hexane. However, the chloride and nitride in 1 were found to be twofold disordered. A model with site occupancies of 0.3 and 0.7 for the two sites was used for the refinement. Fig. 1 shows a perspective view of the molecule; selected bond lengths and angles are listed in Table 2. The Re–P and average Re–O distances were found to be 2.372(1) and 2.183 Å. Oxidation of 1 with AgBF4 afforded [ReLOEtN(PPh3)Cl]BF41·BF4. The measured μeff of 1.8 μB is consistent with the formulation of ReVI. Although the ReVI–ReV couple for 2 is reversible (see later section), no well defined products were obtained for oxidation of 2 with silver(I) salts. The nitride in 1 was found to be nucleophilic and react with electrophiles to give imidorhenium(V) complexes. Thus, treatment of 1 with MeOSO2CF3 afforded the methylimido complex [ReLOEt(NMe)(PPh3)Cl][CF3SO3] 3. The N-methyl protons in 3 were found to couple with the phosphorus of PPh3 and appear as a doublet at δ 1.95 (4JHP = 4.92 Hz). Similarly interaction of 1 with PhCH2Br or [Ph3C]BF4 gave the respective imido species [ReLOEt(NCH2Ph)(PPh3)Cl]Br 4 or [ReLOEt(NCPh3)(PPh3)Cl]BF45. These imidorhenium(V) complexes are air stable in both the solid state and solution.
O) for 2 was not assigned due to the presence of ligand bands in the region. X-Ray quality crystals of complex 1 were obtained by recrystallisation from CH2Cl2–hexane. However, the chloride and nitride in 1 were found to be twofold disordered. A model with site occupancies of 0.3 and 0.7 for the two sites was used for the refinement. Fig. 1 shows a perspective view of the molecule; selected bond lengths and angles are listed in Table 2. The Re–P and average Re–O distances were found to be 2.372(1) and 2.183 Å. Oxidation of 1 with AgBF4 afforded [ReLOEtN(PPh3)Cl]BF41·BF4. The measured μeff of 1.8 μB is consistent with the formulation of ReVI. Although the ReVI–ReV couple for 2 is reversible (see later section), no well defined products were obtained for oxidation of 2 with silver(I) salts. The nitride in 1 was found to be nucleophilic and react with electrophiles to give imidorhenium(V) complexes. Thus, treatment of 1 with MeOSO2CF3 afforded the methylimido complex [ReLOEt(NMe)(PPh3)Cl][CF3SO3] 3. The N-methyl protons in 3 were found to couple with the phosphorus of PPh3 and appear as a doublet at δ 1.95 (4JHP = 4.92 Hz). Similarly interaction of 1 with PhCH2Br or [Ph3C]BF4 gave the respective imido species [ReLOEt(NCH2Ph)(PPh3)Cl]Br 4 or [ReLOEt(NCPh3)(PPh3)Cl]BF45. These imidorhenium(V) complexes are air stable in both the solid state and solution.

![Perspective view of [ReLOEtN(PPh3)Cl] 1.](/image/article/2000/DT/a906618g/a906618g-f1.gif)
![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) N–Au) at 953 cm−1, which is higher than the ν(Re
N–Au) at 953 cm−1, which is higher than the ν(Re![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) for 1. Enhancement of the metal–nitride stretching frequency upon formation of μ-nitride bridges has also been observed for binuclear complexes of Os nitrides, e.g. in [Au(PPh3)(NOsO3)].16 Reaction of 1 with [Ru(Et2dtc)(PPh3)2(CO)(CF3SO3)],14 which was prepared from [Ru(Et2dtc)(PPh3)2(CO)H]
N) for 1. Enhancement of the metal–nitride stretching frequency upon formation of μ-nitride bridges has also been observed for binuclear complexes of Os nitrides, e.g. in [Au(PPh3)(NOsO3)].16 Reaction of 1 with [Ru(Et2dtc)(PPh3)2(CO)(CF3SO3)],14 which was prepared from [Ru(Et2dtc)(PPh3)2(CO)H]![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 13 and triflic acid, afforded [Ru(Et2dtc)(PPh3)(H2O)(CO){NReLOEt(PPh3)Cl}][CF3SO3] 7. One PPh3 dissociated from Ru upon formation of 7 possibly due to steric congestion in the bimetallic complex. A preliminary diffraction study of 7 showed that the aqua ligand, presumably derived from moisture in the solvent, is trans to the CO, and the nitride is trans to a sulfur of Et2dtc as shown below.
13 and triflic acid, afforded [Ru(Et2dtc)(PPh3)(H2O)(CO){NReLOEt(PPh3)Cl}][CF3SO3] 7. One PPh3 dissociated from Ru upon formation of 7 possibly due to steric congestion in the bimetallic complex. A preliminary diffraction study of 7 showed that the aqua ligand, presumably derived from moisture in the solvent, is trans to the CO, and the nitride is trans to a sulfur of Et2dtc as shown below.
![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O stretching frequency for 8 of 920 cm−1 is lower than that for [ReMeO3] (950 cm−1),17 indicating that the Re
O stretching frequency for 8 of 920 cm−1 is lower than that for [ReMeO3] (950 cm−1),17 indicating that the Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O bonds are weakened upon adduct formation. Like 6, the Re
O bonds are weakened upon adduct formation. Like 6, the Re![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching frequency for 8 (953 cm−1) is higher than that for 1.
N stretching frequency for 8 (953 cm−1) is higher than that for 1.![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 18 and NaLOEt possibly because of the preference of OsVI for trans-O
18 and NaLOEt possibly because of the preference of OsVI for trans-O![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) Os
Os![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O geometry. The crystal structure of 9 has been determined and is shown in Fig. 2. Selected bond lengths and angles are listed in Table 3. The geometry around Os is essentially octahedral. The Os–N and average Os–Cl in 9 are 1.58(1) and 2.289 Å, respectively. The Os–O bond that is trans to the nitride (2.159(8) Å) is significantly longer than the other two Os–O bonds (average 2.039 Å), indicative of trans influence of the nitride. The IR Os–N stretching frequency was not assigned due to the presence of ligand bands in the 1000–1100 cm−1 region.
O geometry. The crystal structure of 9 has been determined and is shown in Fig. 2. Selected bond lengths and angles are listed in Table 3. The geometry around Os is essentially octahedral. The Os–N and average Os–Cl in 9 are 1.58(1) and 2.289 Å, respectively. The Os–O bond that is trans to the nitride (2.159(8) Å) is significantly longer than the other two Os–O bonds (average 2.039 Å), indicative of trans influence of the nitride. The IR Os–N stretching frequency was not assigned due to the presence of ligand bands in the 1000–1100 cm−1 region.
![Perspective view of [OsLOEtNCl2] 9.](/image/article/2000/DT/a906618g/a906618g-f2.gif)
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 19 and [OsTpNCl2] (Tp = hydridotris(pyrazol-1-yl)borate),20 complex 9 does not react with nucleophiles such as NaN3, Me3NO and propylene sulfide. Reaction of 9 with PPh3 in CH2Cl2 afforded the osmium(IV) phosphoraniminato complex [OsLOEt(NPPh3)Cl2] 10, which was characterised by X-ray diffraction. Fig. 3 shows a perspective view of 10, selected bond lengths and angles are given in Table 4. The Os–N distance in 10 of 1.893(5) Å is comparable to that in trans-[Os(tpy)Cl2(NPPh3)]+ (2.093(5) Å),21 consistent with the formulation of a Os–N single bond. The P–N bond distance of 1.575(5) Å is similar to that in trans-[Os(tpy)Cl2(NPPh3)]+ (1.618(5) Å)
19 and [OsTpNCl2] (Tp = hydridotris(pyrazol-1-yl)borate),20 complex 9 does not react with nucleophiles such as NaN3, Me3NO and propylene sulfide. Reaction of 9 with PPh3 in CH2Cl2 afforded the osmium(IV) phosphoraniminato complex [OsLOEt(NPPh3)Cl2] 10, which was characterised by X-ray diffraction. Fig. 3 shows a perspective view of 10, selected bond lengths and angles are given in Table 4. The Os–N distance in 10 of 1.893(5) Å is comparable to that in trans-[Os(tpy)Cl2(NPPh3)]+ (2.093(5) Å),21 consistent with the formulation of a Os–N single bond. The P–N bond distance of 1.575(5) Å is similar to that in trans-[Os(tpy)Cl2(NPPh3)]+ (1.618(5) Å)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 21 and is typical for a P
21 and is typical for a P![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) N double bond. As expected, the average Os–Cl distance in 10 (2.342 Å) is longer than that in 9. Unlike most osmium(IV) phosphoran iminato complexes that contain linear Os–N
N double bond. As expected, the average Os–Cl distance in 10 (2.342 Å) is longer than that in 9. Unlike most osmium(IV) phosphoran iminato complexes that contain linear Os–N![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) PR3 moieties,22 the Os–N–P linkage in 10 is bent with an angle of 137.5(3)°. The Os–N–P angle is slightly bigger than that in trans-[Os(tpy)Cl2(NPPh3)]+ (132.5(3)°).21 The measured magnetic moment for 10 of ca. 2.0 μB is different from the spin–only value for two unpaired electrons (2.83 μB) because of spin–orbit coupling.21
PR3 moieties,22 the Os–N–P linkage in 10 is bent with an angle of 137.5(3)°. The Os–N–P angle is slightly bigger than that in trans-[Os(tpy)Cl2(NPPh3)]+ (132.5(3)°).21 The measured magnetic moment for 10 of ca. 2.0 μB is different from the spin–only value for two unpaired electrons (2.83 μB) because of spin–orbit coupling.21![Perspective view of [OsLOEt(NPPh3)Cl2] 10.](/image/article/2000/DT/a906618g/a906618g-f3.gif)
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) °) for Re– and Os–LOEt complexes
°) for Re– and Os–LOEt complexes
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) °
°![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a/V vs. Cp2Fe+/0
a/V vs. Cp2Fe+/0![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b
b![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b
b![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b
b