Direct spectroscopic evidence for the specific property of aqueous core phase in black soap film
Xuezhong
Du
*a and
Yingqiu
Liang
b
aDalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023, China. E-mail: xuezdu@ms.dicp.ac.cn; Fax: 086 0411 4684839
bDepartment of Chemistry and State Key Laboratory of Coordination Chemistry, Nanjing University, Nanjing, 210093, China
First published on 19th January 2000
Abstract
The microenvironment of the aqueous core phase in the black soap film of cationic surfactant cetyltrimethylammonium bromide with the anionic dye Brilliant Yellow as spectral probe has been studied by UV–vis spectroscopy. Under neutral and basic conditions, the dye aggregates in the films exist as both the acid and base forms in contrast to a preference of the base form in the bulk solutions. The specific property of black soap film, that the intrinsic pH value of the aqueous core phase insensitively responds to pH changes of the bulk solution, is directly observed through UV–vis spectra.
Introduction
Black soap film (BSF) possesses a sandwich structure consisting of a thin layer of aqueous core enclosed between two surfactant monolayers,1 and is sufficiently thin in the thickness range 4–50 nm. The molecular orientation of two monolayers in BSFs is precisely the reverse of that in black lipid membranes (BLMs), however, BSFs exhibit several properties similar to BLMs including thickness, refractive index, hydrodynamic behavior, and stability.2 BSFs are simple and well-defined chemical systems that involve the basic physical interaction existing in more complex structures, for example, biological membranes,3 they are thus considered to be important mimetic systems for the understanding of the structure and functions of biomembranes. Dye molecules have been extensively used to gain insight into the characteristics of microenvironments of micelles, membranes, and proteins. When acid–base indicator molecules are adsorbed at the charged interface, the electrostatic interactions between them give rise to structural conversion from one form to the other.4,5 Zhang and Liang6 investigated the BSF of cetyltrimethylammonium bromide (CTAB) with the addition of Brilliant Yellow (BY), an anionic dye, by use of resonance Raman spectroscopy, and discovered the function which was similar to that of biomembranes, that the pH value of the aqueous core phase was insensitive to pH changes of the bulk solution.UV–vis spectroscopy is one of the most convenient and powerful tools for the study of acid–base equilibra of indicator dyes. Interconversions between the acid and base forms pass through an isosbestic point, and two absorption maxima correspond to two structural forms. This work reports direct evidence for the specific property of the aqueous core phase of BSF, instead of depending on a working curve from the Raman spectra of aqueous BY solutions as reported previously.6 Moreover, the high sensitivity of UV–vis spectroscopy and only two component bands of the indicator dye make the experimental result more reliable.
Experimental
Chemicals
The CTAB and BY samples used in the experiment were the same as those described previously.4 The CTAB sample was of spectral grade and was used as received; the BY sample was of analytical grade and was recrystallized from anhydrous ethanol prior to use. The pH values of bulk solutions (10 mM CTAB–1 mM BY) were adjusted with HCl and NaOH (analytical grade) solutions.Measurement
The UV–vis spectra were recorded on a Shimadzu UV 3100 spectrometer at room temperature. The sample cell adopted for the spectral measurements of BSFs was the same as that reported recently.7 A metal frame 7 mm wide and 8 mm high together with a plug on the top of its center axis was used to support a soap film for spectral measurements. Two metal plates with a slit 4 mm high were placed, respectively, in front of the sample and reference cells to prohibit other incident light from passing through. A vertical soap film was lifted up by the frame from the bulk solution, and placed immediately into a quartz cell of 10 mm optical path to wait for the thinning. The aqueous core started to thin by drainage and the soap film exhibited a succession of horizontal interference fringes, then the top of the film became black and the area of the black region gradually expanded until it reached the whole film in 15 min or so. The soap film was preserved through a plateau–Gibbs border, which acted as a container of solution to keep the film stable, between the soap film and the bottom of the supporting frame. The UV–vis spectra of the bulk solutions and surfactant free solutions (0.1 mM) were measured with quartz cells of 0.5 mm thickness.Results and discussion
Fig. 1 shows the chemical structure and acid–base equilibrium of BY. In aqueous solution, the acid–base equilibrium of BY takes place with pK 8.75,4 and the absorption maxima of the conjugated acid and base are located at 400 and 490 nm, respectively (shown in Fig. 2).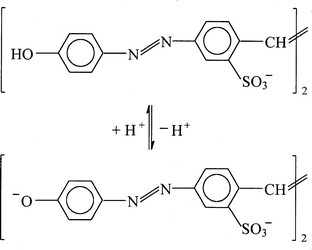 | ||
| Fig. 1 Chemical structure and acid–base equilibrium of BY. | ||
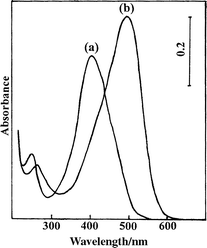 | ||
| Fig. 2 UV–vis spectra of aqueous BY solutions at (a) pH 2.5 and (b) pH 10.0. | ||
Fig. 3 shows the UV–vis spectra of bulk solutions (a) and BSFs (b) and the second derivative spectra of the corresponding BSFs (c) at different pH values of bulk solutions. In Fig. 3(a), at pH 2.5 and pH 6.0, BY molecules pronouncedly take the acid form (380 nm) on the surface of cationic CTAB micelles, while at pH 6.7 and pH 7.2, the base form of BY molecules (460 nm) is preferred. The two absorption maxima are blue shifted in comparison to those in aqueous solution, which indicates that the BY molecules are H-type aggregates in which the head-to-head orientation of the chromophores is formed on the positively charged surface in both acid and base forms.8 It can be easily seen that the cationic micelles shift the acid–base equilibrium of BY toward the base form.
 | ||
| Fig. 3 UV–vis spectra of BY in (a) bulk solutions and (b) BSFs, and (c) the second derivative spectra of BSFs at different pH values of bulk solutions. | ||
In Fig. 3(b), at pH 2.5 and pH 6.0, the BSFs show absorption maxima at approximately 360 nm, implying that the dye molecules are more close packed than those on the micellar surface. When the pH value of the bulk solution is increased to 6.7, the BY molecules in the film have a preference for the acid form in contrast to a dominant base form populated on the micellar surface. Moreover, the second derivative spectrum of the film in Fig. 3(c) exhibits a single negative peak at 360 nm as shown in the cases of pH 2.5 and pH 6.0, suggesting that the aggregate of acid fashion is formed. Upon a further increase of the pH to 7.2, the spectrum of the BSF shows a broad band profile and the corresponding derivative spectrum displays two negative peaks at 360 and 460 nm with comparable intensities. The 460 nm band may be assigned to the base form of the BY aggregate in the films. In the vicinity of the neutral condition (pH 6.7 and pH 7.2), BY in the bulk solutions is predominantly of the base form while in the BSFs BY primarily exhibits the characteristics of the coexistence of the acid and the base forms. The pH change of the aqueous core phase of the BSF exhibits a hysteresis phenomenon in comparison with that of the corresponding bulk solution. The fact that the acid–base equilibrium of BY in the aqueous core phase of the BSF insensitively responds to pH changes of the bulk solution is directly observed from the UV–vis spectra. The aqueous core phase of the film shows a buffer effect to a certain extent against the bulk solution, which is consistent with the result deduced by resonance Raman spectroscopy.6
In both concentrated acidic and basic bulk solutions, the absorbances of BY have been increased substantially so that the intensities are beyond the limit of detection, therefore it is impossible to obtain the UV–vis spectra of the bulk solutions containing 1 mM BY in these cases.
Fig. 4 shows the UV–vis spectra of 10 mM CTAB–1 mM BY BSFs at different pH values of bulk solutions. At pH 1.0, 2.5, 6.0, and 6.7, the BY aggregates take the acid form in the interface of the aqueous phases of the films. The decrease of the 360 nm band intensity is correlated to the pH variation of the bulk solutions. While at pH 7.2, 8.2, and 9.0, the acid and the base forms of the dye molecules coexist in the interface of the aqueous phases of the films, characterized by band overlap, and the acid component gradually attenuates with increasing pH value of the bulk solution. Up to pH 10.0, BY in the film primarily takes the base form. As mentioned above, under the neutral and basic conditions (within a large pH region of 6.0–10.0 of bulk solutions), BY molecules take the characteristics of the coexistence of acid and base forms, indicating a specific property of thin layer water in BSFs.
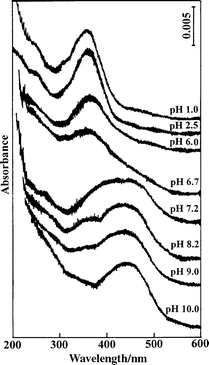 | ||
| Fig. 4 UV–vis spectra of BSFs at different pH values of bulk solutions. | ||
The acid–base equilibrium of BY with structural interconversion is affected by the interfacial electrical potential. Much research has been directed towards determining the electrical potential at the surface.9–12 It has been accepted that on charged membranes, including soap film with a thick layer of water (75–550 nm),11 which have a semi-infinite dispersion layer compared with the Debye-length, the intrinsic pH is directly decided by the electrical potential and the bulk pH according to Boltzmann's law. For BSFs, however, the thickness of the aqueous core (6.5–20 nm)13 approaches or is even smaller than the Debye-length and the specific interaction between the electrical double layer may result in the formation of a relatively stable microenvironment, which is different from that on the micellar surface. The stable microenvironment retains a relatively fixed acidity so that the film exhibits the specific property that the intrinsic pH of the aqueous core phase is insensitive to pH changes of the bulk solution.
Acknowledgements
This work is financially supported by a major research project grant from the State Science and Technology Commission of China.References
- I. B. Ivanov, Thin Liquid Film, Dekker, New York, 1988. Search PubMed.
- Z. I. Lalchev, P. J. Wilde, A. R. Mackie and D. C. Clark, J. Colloid Interface Sci., 1994, 167, 80 CrossRef CAS.
- O. Bélorgey and J. J. Benattar, Phys. Rev. Lett., 1991, 66, 313 CrossRef CAS.
- Y. Zhang and Y. Liang, Huaxue Xuebao, 1993, 51, 586 Search PubMed.
- S. P. Moulik, B. K. Paul and D. C. Mukherjee, J. Colloid Interface Sci., 1993, 161, 72 CrossRef CAS.
- Y. Zhang and Y. Liang, J. Chem. Soc., Chem. Commun., 1994, 1483 RSC.
- X. Du, Y. Lu and Y. Liang, J. Colloid Interface Sci., 1998, 207, 106 CrossRef CAS.
- E. G. McRae and M. Kasha, Physical Processes in Radiation Biology, Academic Press, New York, 1964, p. 23. Search PubMed.
- P. Mukerjee and K. Banerjee, J. Phys. Chem., 1964, 68, 3567 CAS.
- M. S. Fenáderz and P. Fromherz, J. Phys. Chem., 1977, 81, 1755 CrossRef.
- P. Fromherz and R. Kotulla, Ber. Bunsen-Ges. Phys. Chem., 1984, 88, 1106 Search PubMed.
- C. J. Drummond, F. Grieser and T. W. Healy, J. Phys. Chem., 1988, 92, 2604 CrossRef CAS.
- Z. Zhang and Y. Liang, J. Colloid Interface Sci., 1995, 169, 220 CrossRef CAS.
| This journal is © the Owner Societies 2000 |
