Morphology and twin plane structure of silver bromide rod crystals
A. Millan†*ab, N. Dewitc and C. Van Roostc
aRIM, Laboratory of Solid State Chemistry, University of Nijmegen, Toernooiveld, 6525 ED, Nijmegen, The Netherlands
bCondensed Matter Physics, ICMA, CSIC and Universidad de Zaragoza, 50009, Zaragoza, Spain
cAgfa-Gevaert N.V., Septestraat 27, 2640, Mortsel, Belgium
First published on UnassignedUnassigned22nd December 1999
Abstract
Large silver bromide rod crystals have been grown in a solution of silver bromide and potassium bromide in dimethyl sulfoxide. Samples of these crystals were examined by scanning electron microscopy in order to study their side and top face structure. Crystal sides were bounded by {111} and {100} faces. Rod crystals grown from concentrated solutions developed a well-defined top face structure composed of alternating {111} and {100} faces. However, they showed a relatively small length to thickness ratio. On the other hand, top faces of fast growing rod crystals were rough. Slices of rod crystals were examined by transmission electron microscopy to study their twin plane structure, for the first time. All the rod crystals examined showed non-parallel twin planes of (111) type. Surprisingly, all of them also showed parallel twin planes. Rod crystals can transform into tabular or tetrahedral crystals either by formation of new twin planes, or by overgrowth of some twin variants over others in processes that start at the tips of the rod.
Introduction
Silver halide crystals exhibit a large variety of shapes.1–7 Generally speaking, crystal shape is the result of a sum of factors including: relative surface energy of crystal faces, twin planes, dislocations, type of solvent, impurities, kinetic effect, etc. In the case of silver halides, a variation of the first two factors is just enough to originate a wide scope of crystal habits. The most frequent crystal forms in silver halides are {100}, {110} and {111} as it should be expected from their face centred cubic (fcc) structure.8 Other crystal forms such as {331}, {211}, {210}, {410}, {321} . . . have also been found in silver bromide crystals.6,7 On the other hand, silver halide crystals may form single and multiple twins. The natural composition plane in these twins is (111).2–5 Other twin planes observed in these crystals, such as (221), (411), (511),3 seem to be originated by contact of growing crystals rather than by real twinning events on a crystal face of the same orientation.An
interesting habit among silver halide crystals is the elongated
habit. Hamilton and Brady2
considered silver halide rod
crystals as the result of non-parallel multiple twinning on
(111) faces. Klein et al.3 described several types of silver
bromide and silver chloride rod crystals bounded by {111}
faces, {100} faces, or both. These authors proposed several
twin plane structures for rod crystals consisting of 2, 3, 4 or 5
non-parallel twins of the (111) type and one twin plane of the
(221), (411), (511), (877) or (11111) type. Goessens et al.9 examined
silver bromide needle crystals by electron diffraction and
found three twin variants. The first one was attached through
(111) planes to the other two, which were meeting on a (22![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) plane. Assuming a {111} crystal morphology they derived a face structure depicted in Fig. 1. Ridges and troughs on the rod tops were claimed as responsible for accelerated length growth. Real observation of side and top faces was hindered at
that time by the small size of the crystals and the unavoid
able
deformations caused on the crystals during the gelatin separation process. A new method allows now a production of large rod crystals in a short time in the absence of gelatin.10
Using this method, Bögels et al.11 were able to measure dihedral
angles between lateral faces with a goniometer. They assumed a twin plane structure coincident with that proposed by Goessens et al. However, they proposed a different top face structure that included (100) faces. They also observed silver chloride rod crystals grown from the vapor phase and they found (100) faces on the rod tops. They proposed a very complex twin plane structure for these crystals consisting of 8 or 9 twin planes of the (111) type. Using this new method, we were able to observe the growth of needle crystals in situ by optical microscopy.12 It was found that needle crystals were transformed along precipitation in three different ways: some
of them
dissolved, some were converted into tabular crystals and
others were converted into tetrahedral crystals.
) plane. Assuming a {111} crystal morphology they derived a face structure depicted in Fig. 1. Ridges and troughs on the rod tops were claimed as responsible for accelerated length growth. Real observation of side and top faces was hindered at
that time by the small size of the crystals and the unavoid
able
deformations caused on the crystals during the gelatin separation process. A new method allows now a production of large rod crystals in a short time in the absence of gelatin.10
Using this method, Bögels et al.11 were able to measure dihedral
angles between lateral faces with a goniometer. They assumed a twin plane structure coincident with that proposed by Goessens et al. However, they proposed a different top face structure that included (100) faces. They also observed silver chloride rod crystals grown from the vapor phase and they found (100) faces on the rod tops. They proposed a very complex twin plane structure for these crystals consisting of 8 or 9 twin planes of the (111) type. Using this new method, we were able to observe the growth of needle crystals in situ by optical microscopy.12 It was found that needle crystals were transformed along precipitation in three different ways: some
of them
dissolved, some were converted into tabular crystals and
others were converted into tetrahedral crystals.
 | ||
Fig. 1
Ideal top face structure of a silver bromide rod crystal. It is bounded by {111} faces, and it has two twin planes of (111) type and a third one of (22![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) type. ) type. | ||
In this work, we have grown large rod crystals in a KBr–AgBr–DMSO system and we have observed their side and top face structure by scanning electron microscopy. We have also cut ultra thin slices of rod crystal samples and we have observed their internal twin plane structure by transmission electron microscopy. The results of this work permit a better understanding of the mechanisms of growth of multiple twin crystals.
Experimental
Silver bromide crystals were precipitated from solutions of KBr and AgBr in DMSO by addition of water. The concentration of silver in the precipitating solution ranged from 0.1 to 2 mol L−1. Some samples were precipitated on a microscope slide under observation by optical microscopy before being separated from the mother phase. Other samples were produced in a flask and they were kept in the precipitating medium for several days before separation.Optical microscopy observations were carried out on a Nikon microphot-FX microscope. Scanning electron microscopy (SEM) observations were performed on a Jeol JSM-T300 instrument. Ultra-thin slices of crystal samples were cut with a diamond knife (Reichert Jung) and examined with a Philips EM 400 transmission electron microscope (TEM).
Results and discussion
Side and top face structure of rod crystals
Previous works showed that the morphology of crystals precipitated from KBr–AgBr–DMSO solutions varies with the concentration of silver in the solution.7,10 Crystals from concentrated solutions are mainly bounded by the {100} and {110} forms. But, as concentration decreases, the importance of the {111} form increases. Below a concentration of about 0.1 mol L−1, the {111} form is the only one present. That applies to rod crystals also. Fig. 1 shows SEM images of thick rod crystals grown in a flask from solutions with a 1 M silver concentration. They have a well-defined morphology on sides and tops that varies among different crystals. The side and top face structure of the crystal in Fig. 2A can be easily identified by comparison with the type I crystal in Fig. 6 that was drawn with the help of the programme SHAPE. Twin planes, marked by arrows in the picture, correspond with the structure found by Goessens et al.9 The lateral face structure consists of (111) faces as proposed in that work. However, the top face structure differs from the fact that {100} faces are also present together with predicted (111) faces. These faces are arranged alternatively creating a hollow region at the confluence of the three twin planes. Other faces with an approximate (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) orientation were
occasionally observed (black spot on Fig. 2B). The crystal
in Fig. 2C shows a more complicated twin plane structure.
The groove shown by an arrow in the picture is indicating the presence of a 3rd (111) twin plane in the crystal. Apparently, this rod crystal was extending laterally along its rough side. The most common face structure found on well-developed
rod crystals was that of the crystal in Fig. 2D. This crystal as well as those in Figs. 2E and F was grown from a 0.5 M silver solution. It is flattened as to resemble a trapezoid. Its top face structure is simple and consists of two (111) faces like in triangular tabular crystals.7 This basic shape could become more
complex by formation of additional twin variants as in the crystal in Fig. 2E. Some rod crystals had rhombic shape instead of trapezoidal such as crystal 1 in Fig. 2F. In this crystal, the top right part is bounded by (111) faces while the top left part is bounded by two small (111) faces (upper part) and two large (100) faces (lower part). On the other hand, crystal 2 in Fig. 2F has the shape of a triangular prism.
) orientation were
occasionally observed (black spot on Fig. 2B). The crystal
in Fig. 2C shows a more complicated twin plane structure.
The groove shown by an arrow in the picture is indicating the presence of a 3rd (111) twin plane in the crystal. Apparently, this rod crystal was extending laterally along its rough side. The most common face structure found on well-developed
rod crystals was that of the crystal in Fig. 2D. This crystal as well as those in Figs. 2E and F was grown from a 0.5 M silver solution. It is flattened as to resemble a trapezoid. Its top face structure is simple and consists of two (111) faces like in triangular tabular crystals.7 This basic shape could become more
complex by formation of additional twin variants as in the crystal in Fig. 2E. Some rod crystals had rhombic shape instead of trapezoidal such as crystal 1 in Fig. 2F. In this crystal, the top right part is bounded by (111) faces while the top left part is bounded by two small (111) faces (upper part) and two large (100) faces (lower part). On the other hand, crystal 2 in Fig. 2F has the shape of a triangular prism. | ||
| Fig. 2 Thick rod crystals precipitated from KAgBr2 solutions in DMSO. Bar at the bottom corresponds to 10 μm. A, B and C, crystals grown in a flask during 2 weeks from 1 M solutions; D, E and F, crystals grown on a microscope slide during 6 h from 0.5 M solutions. | ||
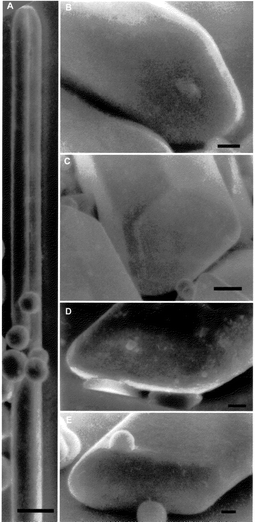
Rod crystals were grown on a microscope slide under optical microscopy observation from solutions with a 0.1–0.2 M silver concentration. They showed fast elongation rate and a rough top surface, in the early stages of growth. Fig. 3A shows a typical needle-crystal with a completely rounded top. One of the twin variants of this crystal was lost after the adherence of small rounded crystals. Frequent features on the tops of thin needle crystals are the presence of hollow regions and acute points (Figs. 3B and D). Needle crystals from diluted solutions also developed some faces on the tops after long growing periods. The crystal in Fig. 3C shows incipient (111) faces on the top point. The crystal in Fig. 3E has already developed {111} faces in a convex disposition along the whole top. Thereafter, this type of rod crystals extended laterally transforming into tabular crystals (Fig. 4A). However, other rod crystals developed a unique (111) plane at each top (crystal 2 in Fig. 2F) and they transformed into tetrahedral crystals (Figs. 4B–E).
 | ||
| Fig. 3 Thin rod crystals precipitated on a microscope slide from 0.1 M KAgBr2 solutions in DMSO. Bar at the bottom corresponds to 1 μm. | ||
 | ||
| Fig. 4 Rod crystals being transformed into: A, triangular tabular crystal; B, C and D tetrahedral crystal. Bar at the bottom is 10 μm. | ||
Ultra thin slices of rod crystal samples were examined by
TEM. The results are presented in Fig. 5. All the crystals
examined have non-parallel twin planes and, surprisingly, parallel twin planes also. Furthermore, the angle between non-parallel twin planes is about 70°. That corresponds to faces (111) and (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11) sharing a corner, while, crystals in Figs. 2A–C
and the model proposed by Goessens et al.9 showed non-parallel twinning on (111) and (
11) sharing a corner, while, crystals in Figs. 2A–C
and the model proposed by Goessens et al.9 showed non-parallel twinning on (111) and (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11) faces, which share an edge. Another remarkable feature is the presence of (100) lateral faces. Thus, rod crystals may have two different types of twin plane structure represented in Fig.
6. Fig. 5A shows a slice of a thin rod crystal grown for two hours in a 0.1 M silver
solution. This can be taken as the basic structure of rod crystals of type II (Fig. 6). In the rod crystal in Fig. 5B, twin variant 2 is covering the bottom side of twin variant 4. That may correspond to a form of evolution from the basic structure. A further stage of development is revealed in Fig. 5C. It shows a slice of another rod crystal cut near the top. Twin variant 3 has been partially overgrown by twin variants 1 and 4 that
have the same crystallographic orientation. The rod crystal in Fig. 5D shows a more complex way of evolution from the basic structure. This crystal presents several additional
twin boundaries and two acute edges that correspond to fronts of lateral extension of the crystal. This side geometry is strange considering the twin plane structure of the crystal
because
(111) faces can be formed at those sites. However, in situ
observations showed that changes of shape on rod crystals originated at the tops. Therefore, the presence of acute dihedral angles on the sides of this crystal may obey a growth event occurring on the top of the rod. Fig. 5F shows the twin plane structure near the tip of this crystal. The two twin structures do not match indicating the formation of a new twin variant at the top. Fig. 5E is of a very bad quality because the crystal was partially destroyed by the electron beam before taking
the picture. However, we have considered it interesting to include it because it shows another way of transformation of a rod crystal into a tabular crystal. In this case, an original rod crystal of type II developed three new twin planes parallel to the
(1
11) faces, which share an edge. Another remarkable feature is the presence of (100) lateral faces. Thus, rod crystals may have two different types of twin plane structure represented in Fig.
6. Fig. 5A shows a slice of a thin rod crystal grown for two hours in a 0.1 M silver
solution. This can be taken as the basic structure of rod crystals of type II (Fig. 6). In the rod crystal in Fig. 5B, twin variant 2 is covering the bottom side of twin variant 4. That may correspond to a form of evolution from the basic structure. A further stage of development is revealed in Fig. 5C. It shows a slice of another rod crystal cut near the top. Twin variant 3 has been partially overgrown by twin variants 1 and 4 that
have the same crystallographic orientation. The rod crystal in Fig. 5D shows a more complex way of evolution from the basic structure. This crystal presents several additional
twin boundaries and two acute edges that correspond to fronts of lateral extension of the crystal. This side geometry is strange considering the twin plane structure of the crystal
because
(111) faces can be formed at those sites. However, in situ
observations showed that changes of shape on rod crystals originated at the tops. Therefore, the presence of acute dihedral angles on the sides of this crystal may obey a growth event occurring on the top of the rod. Fig. 5F shows the twin plane structure near the tip of this crystal. The two twin structures do not match indicating the formation of a new twin variant at the top. Fig. 5E is of a very bad quality because the crystal was partially destroyed by the electron beam before taking
the picture. However, we have considered it interesting to include it because it shows another way of transformation of a rod crystal into a tabular crystal. In this case, an original rod crystal of type II developed three new twin planes parallel to the
(1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1) face of twin variant 2. The advancement front for lateral
extension is a rough acute edge. In all the cases examined of
rod crystals of type II, the separation between parallel twin planes is quite narrow as compared with the dimensions of the rest of the twin variants. This separation is similar to that found in tabular crystals from the same precipitate. It is likely that rods of type II are originated from parallel twin crystals.
1) face of twin variant 2. The advancement front for lateral
extension is a rough acute edge. In all the cases examined of
rod crystals of type II, the separation between parallel twin planes is quite narrow as compared with the dimensions of the rest of the twin variants. This separation is similar to that found in tabular crystals from the same precipitate. It is likely that rods of type II are originated from parallel twin crystals.
 | ||
| Fig. 5 Electron microscope images of ultrathin slices of rod crystals. | ||
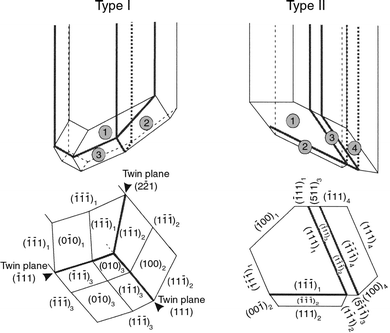 | ||
| Fig. 6 Main types of rod crystals. Type I, non-parallel twinning on faces sharing an edge. Type II, non-parallel twinning on faces sharing a corner. | ||
Next, we can try to explain the growth of rod crystals from
their morphology and twin plane structure. We have pointed
out above that crystals with a high length to thickness ratio
are preferentially grown from diluted solutions. Observations
in situ of crystal growth and SEM observations on thin needle
crystals indicate that fast elongation rates correspond to crystals
with flat lateral faces and rough tops. Elongation growth
on these crystals can be easily understood because rough faces
grow obviously faster than flat faces. However, why are flat
faces initially developed at the sides and not at the tops? It is
probably the formation of twin planes that precludes the formation
of flat lateral faces by extension of the new twin
variants along the twin planes. Then, a non-parallel twin plane structure produces the formation of prismatic faces parallel to the twin faces, whereas the tops remain rough. Besides, the twin plane structure of rod crystals impose a complex top face geometry that can only be constructed by formation of re-entrant angles, acute dihedral angles and (100) faces. Once a crystal has developed an elongated shape, another factor is reinforcing length growth. That is because the diffusion field is far more intense around the tops of a needle than on the sides. Thus, crystal tops will remain rough and grow at a high rate while the transport of ions from the bulk to the crystal surface is fast enough. As concentration decreases, the rate of transport of add-atoms onto the surface may
decrease below the rate of ion surface diffusion. Then, rod crystal tops might develop flat faces. The evolution of a rod crystal afterwards depends on the type of rod and the medium. In concentrated media with a low Br−/Ag+ ratio, (100)
faces are stable.7,10,12 Then, rod crystals can develop a full morphology consisting mainly of (111) and (100) faces. In media
with a low concentration of silver and high Br−/Ag+
ratio, (100) faces are unstable.10 Then, rod crystals dissolve or they are
transformed into triangles or tetrahedra.12 Sides of rod crystals of type I are bounded by stable (111) faces. But the tops
can only develop a (111) face structure with re-entrant angles. It seems such morphology is not very stable in diluted solutions because we have never observed it. Probably, dissolving rod crystals belong to this type. Observations by SEM has shown that two (111) faces in a convex disposition bound the
tops of crystals transforming into triangles. That is consistent with TEM observations indicating that overgrowth of the middle twin variant can leave only two twin variants at the top. Thus, the transformation of rod crystals into triangles can take place as depicted in Fig. 7A. In step 1, twin variant 2 of the basic structure extends toward the end of the right side (compare with the crystal in Fig. 4B). In step 2, twin variants 2 and 4 begin to develop (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) and (
) and (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11) faces respectively at the tip of the rod (Fig. 3C). In step 3, the extension of these faces over the crystal top covers up twin variant 3, which is normally sunken
(Figs. 3B and D). This operation leaves the crystal with
three sides bounded by (111) faces in a stable disposition and
a fourth side bounded by an unstable (100) face (Fig. 5C). In step 4, the crystal extends perpendicularly to the (100) face until it becomes a triangular crystal bounded exclusively by (111) faces (Figs. 3E and 4A). Nevertheless, the formation of tabular crystals from rod crystals can take place in other ways as it is shown in Fig. 5E. The mechanism of transformation of
rod crystals into tetrahedral crystals is not so clear. Our observations indicate two different ways of transformation. Longitudinal grooves on the crystal in Fig. 4C suggest a twin plane structure as depicted in Fig. 7B. We did not observed
this twin structure by TEM but it is consistent with the twin structure proposed for tetrahedral crystals by Klein et al.3
The evolution of this crystal to become a tetrahedron could
be as follows: (1′) development of (111) top faces on twin variants 2 and 3; (2′) development of (111) top faces on twin variant 1; (3′) extension of twin variants 2 and 3 along
lateral (111) faces
until a tetrahedron is formed. There is a second way of transforming a rod crystal into a tetrahedron represented in Fig. 7C. This crystal does not show any grooves, its top face is completely flat, and its middle part is sunken. The shape of this crystal is consistent with the twin plane structure
of crystal in Fig. 5D. It was pointed out above that a feature of the rod top must determine the rare side geometry shown by this crystal. Actually, the extension of rod crystals along two planes begins at the tops (Fig. 4B). This feature can be the formation of a new twin on the (
11) faces respectively at the tip of the rod (Fig. 3C). In step 3, the extension of these faces over the crystal top covers up twin variant 3, which is normally sunken
(Figs. 3B and D). This operation leaves the crystal with
three sides bounded by (111) faces in a stable disposition and
a fourth side bounded by an unstable (100) face (Fig. 5C). In step 4, the crystal extends perpendicularly to the (100) face until it becomes a triangular crystal bounded exclusively by (111) faces (Figs. 3E and 4A). Nevertheless, the formation of tabular crystals from rod crystals can take place in other ways as it is shown in Fig. 5E. The mechanism of transformation of
rod crystals into tetrahedral crystals is not so clear. Our observations indicate two different ways of transformation. Longitudinal grooves on the crystal in Fig. 4C suggest a twin plane structure as depicted in Fig. 7B. We did not observed
this twin structure by TEM but it is consistent with the twin structure proposed for tetrahedral crystals by Klein et al.3
The evolution of this crystal to become a tetrahedron could
be as follows: (1′) development of (111) top faces on twin variants 2 and 3; (2′) development of (111) top faces on twin variant 1; (3′) extension of twin variants 2 and 3 along
lateral (111) faces
until a tetrahedron is formed. There is a second way of transforming a rod crystal into a tetrahedron represented in Fig. 7C. This crystal does not show any grooves, its top face is completely flat, and its middle part is sunken. The shape of this crystal is consistent with the twin plane structure
of crystal in Fig. 5D. It was pointed out above that a feature of the rod top must determine the rare side geometry shown by this crystal. Actually, the extension of rod crystals along two planes begins at the tops (Fig. 4B). This feature can be the formation of a new twin on the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1
1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) face of twin variants 1 or 4 (step 2″). The new twin variant would grow across the rod top covering up the rest of the twin variants (step 3″; see the crystal in Fig. 5B). The geometry of the crystal would be determined by the extension of the new twin variant toward
the centre of the crystal (step 4″). Observe that the advancement
front of the crystal in Fig. 5D is composed of a unique twin variant.
) face of twin variants 1 or 4 (step 2″). The new twin variant would grow across the rod top covering up the rest of the twin variants (step 3″; see the crystal in Fig. 5B). The geometry of the crystal would be determined by the extension of the new twin variant toward
the centre of the crystal (step 4″). Observe that the advancement
front of the crystal in Fig. 5D is composed of a unique twin variant.
 | ||
| Fig. 7 Suggested mechanisms of transformation of rod crystals. A, transformation into a triangular tabular crystal, B and C transformation into tetrahedral crystals. | ||
A recurrent event in this work is the presence of rough faces or acute dihedral angles in the direction of accelerated growth. Parallel twin planes do not necessarily promote accelerated growth. Actually, those crystal sides having parallel twin planes initiating on them seemed to grow slowly in the rod crystals examined. The role of twin planes in growth acceleration might consist of the generation of rough faces or acute dihedral angles on crystal sides. The phenomena of accelerated elongation rate on rod crystals can still be considered from another point of view, that of crystal structure aniso-tropy. Certainly, observations of AgBr dendrite formation have shown a preferential growth along acute edges and corners, which in crystals bounded by {111} faces coincide with the [100] crystallographic direction.7 Growth anisotropy on AgBr crystals is also apparent in the formation of hollow crystals observed in some experiments.13 It must be taken into account that a full face development of rod crystals would lead to the formation of several corners at the rod ends. Thus, growth anisotropy assisted by a high concentration of silver halide complexes in the media could be a cooperating mechanism for accelerated elongation growth.
Acknowledgements
Financial and technical support from Agfa-Gevaert is gratefully acknowledged.References
- T. H. James, The theory of the photographic process, Macmillan, New York, 4th edn., 1977. Search PubMed.
- J. F. Hamilton and L. E. Brady, J. Appl. Phys., 1964, 35, 414 CAS.
- VonE. Klein, H. J. Metz and E. Moisar, Mitteilungen aus den Forschungslaboratorien der Agfa-Gevaert AG Band IV, Springer-Verlag, Berlin, 1964, p. 64. Search PubMed.
- R. W. Berriman and R. H. Herz, Nature, 1957, 180, 293 CAS.
- C. R.Berry and D. C. Skillman, Photogr. Sci. Eng., 1962, 6, 159 Search PubMed.
- J. E. Maskasky, J. Imaging Sci., 1986, 30, 247 Search PubMed.
- A. Millan, P. Bennema, A. Verbeeck and D. Bollen, J. Cryst. Growth, 1998, 192, 215 CrossRef CAS.
- J. D. H. Donnay, Crystal Data. Determinative Tables, American Crystallography Association, New York, 1963. Search PubMed.
- C. Goessens, D. Schryvers, J. Van Laduyt, A. Millán and R. De Keyzer, J. Cryst. Growth, 1995, 151, 335 CrossRef CAS.
- A. Millan, J. Cryst. Growth, 1999, accepted. Search PubMed.
- G. Bögels, T. M. Pot, H. Meekes, P. Bennema and D. Bollen, Acta Crystallogr., Sect. A, 1997, 53, 84 CrossRef.
- A. Millan, P. Bennema, A. Verbeeck and D. Bollen, J. Chem. Soc., Faraday Trans., 1998, 94, 2195 RSC.
- G. Bögels, PhD Thesis, University of Nijmegen, Nijmegen, Holland, 1999..
Footnote |
| † Present address: ICMA, CSIC and Universidad de Zaragoza, 50009 Zaragoza, Spain. E-mail: amillan@posta.unizar.es |
| This journal is © the Owner Societies 2000 |
