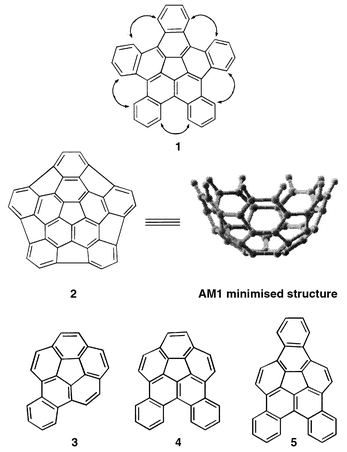
Bucky-bowls. A general approach to benzocorannulenes: synthesis of mono-, di- and tri-benzocorannulenes
Goverdhan
Mehta
* and
P. V. V.
Srirama Sarma
Department of Organic Chemistry, Indian Institute of Science, Bangalore, 560 012, India.. E-mail: diroff@admin.iisc.ernet.in
First published on 24th December 1999
Abstract
We outline a conceptually simple and general route to bowl-shaped benzocorannulenes based on readily assembled PAHs which on flash vacuum pyrolysis result in the sequential formation of a five- and six-membered ring; following this approach, syntheses of mono-, di- and tri-benzocorannulenes have been achieved.
As a part of our continuing interest in the synthesis of C60 fullerene (bucky-ball) and its fragments (bucky-bowls),1,2 we became interested in developing a synthetic approach to pentabenzocorannulene 1en route (see transannular bridging indicated in 1) to the ‘deep-bowl’ 2, C40H10.3 Bowl-shaped 1 and 2 represent 2/3 of the carbon content of C60 with eleven and sixteen rings, respectively, constituting a dominant cross-section on the fullerene surface. Both 1 and 2 evoke considerable synthetic interest and are formidable objectives. As a prelude to efforts towards 1 and 2, we have developed a new and general synthetic route to benzoannulated corannulenes in which an appropriately constructed aromatic array upon flash vacuum pyrolysis (FVP) undergoes two-fold C–C bond formation involving cyclodehydrogenation to generate a five-membered ring, followed by insertion of vinylidene carbene or equivalent species to form a six-membered ring. Herein, we report the synthesis of mono-, di- and tri-benzocorannulenes 3–5.
Our approach to benzocorannulene 3 emanated from 13- methylbenzo[g]chrysene 7, readily available from 9-methyl- phenanthrene 6 through a tactical modification of the reported procedure.4 The methyl group in 7 was oxidised to the required aldehyde 9 in two steps via the intermediate bromide 8 (Scheme 1). The aldehyde functionality in 9 was then elaborated to 10–12 having active functionalities, which on thermal activation under FVP conditions were expected to result in the projected two-fold cyclization. Indeed, FVP of 10–12 furnished 3, albeit in low yields characteristic of such reactions (Scheme 2).2 Benzocorannulene 3 was readily identified through its spectral characteristics (UV, 2D NMR, mass).5,6
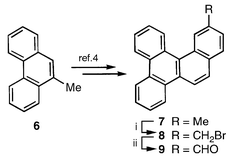 | ||
| Scheme 1 Reagents and conditions: i, NBS, AIBN, CCl4, 73%; ii, (Bu4N)2Cr2O7, CHCl3, 76%. | ||
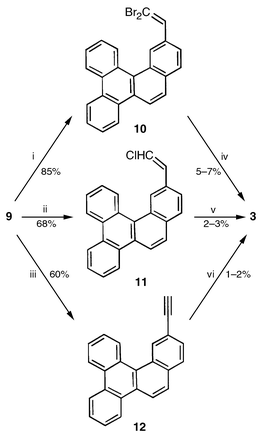 | ||
| Scheme 2 Reagents and conditions: i, CBr4, PPh3, Zn, CH2Cl2, 85%; ii, ClCH2PPh3Cl, ButOK, 0.5 h, 68%; iii, ClCH2PPh3Cl, ButOK, 2 h, 60%; iv, FVP, 1150 °C, 5–7%; v, FVP, 1150 °C, 2–3%; vi, FVP, 1150 °C, 1–2%. | ||
Our approach to dibenzocorannulene 4 originated from 5-methylbenzo[c]phenanthrene 13, in turn readily accessible from commercial 2-methylnaphthalene.7 Naphthoannulation on 13 through the intermediacy of the Wittig salt 14 and photocyclization of the resulting stilbene derivative 15 led to 13-methyldibenzo[c,p]chrysene 16 (Scheme 3). The methyl group in 16 was oxidised to the aldehyde 17 and in the light of the relatively more efficient conversion 10→3 was further transformed to the hexacyclic gem-dibromoalkene 18, the desired FVP precursor. On thermal activation 18 underwent the expected double cyclization to furnish the new dibenzocorannulene 4 and was fully characterised on the basis of incisive spectral analyses5 (Scheme 3).
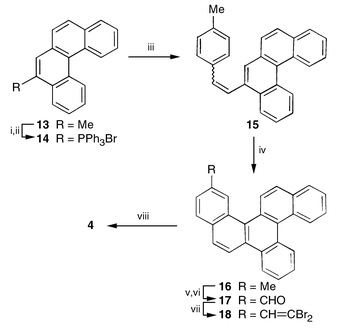 | ||
| Scheme 3 Reagents and conditions: i, NBS 99%; ii, PPh3, C6H6, 79%; iii, p-MeC6H4CHO, Cs2CO3, PriOH, 80%; iv, hv, I2, C6H6, propylene oxide, 65%; v, NBS, CCl4, 44%; vi, (Bu4N)2Cr2O7, CHCl3, 77%; vii, CBr4, PPh3, Zn, CH2Cl2, 85%; viii, FVP, 1150 °C, 5–7%. | ||
Interestingly, 5-methylbenzo[c]phenanthrene 13 and the Wittig salt 14 derived from it also served as the precursor for the synthesis of tribenzocorannulene 5. Wittig coupling between 14 and 4-methylnaphthaldehyde gave 19 which on photocyclization led to the naphtho[1,2-f]picene derivative 20 (Scheme 4). The methyl group in 20 was again elaborated to the aldehyde 21 and further to the FVP precursor 22. As planned, FVP on 22 furnished the desired tribenzocorannulene 5, which was spectroscopically characterised (Scheme 4).5,6
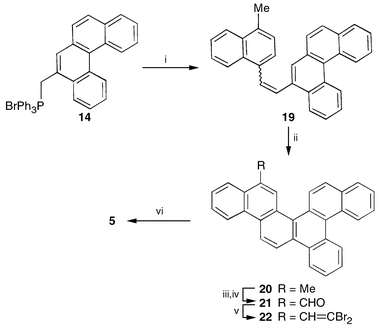 | ||
| Scheme 4 Reagents and conditions: i, 4-methylnaphthaldehyde, Cs2CO3, PriOH; ii, hv, I2, C6H6, propylene oxide, 50% (2 steps); iii, NBS, CCl4, 45%; iv, (Bu4N)2Cr2O7, CHCl3, 70%; v, CBr4, PPh3, Zn, CH2Cl2, 55%; vi, FVP, 1150 °C, 1–2%. | ||
In short, we have accomplished the syntheses of bowl-shaped benzocorannulenes 3–5 from appropriate polycyclic aromatics employing FVP as the key step, in which a five- and a six-membered rings are sequentially formed. The precursor polycyclic platforms were assembled from simple aromatic starting materials through an iterative sequence involving Wittig olefination and photocyclization steps. Notwithstanding the low yields in the final FVP step, which is not uncommon for such cyclizations,1,2 this work demonstrates the generality of our approach and sets the stage for the synthesis of 1 and 2.
Acknowledgements
We thank JNCASR for financial support and the SIF facility at I.I.Sc for high field NMR data. One of us (P. V. V. S. S.) thanks CSIR for a research fellowship. We thank Professor L. T. Scott for generously providing copies of spectra for comparison purposes.References
- (a) R. Faust, Angew. Chem., Int. Ed. Engl., 1995, 34, 1429 CrossRef CAS; (b) L. T. Scott, Pure Appl. Chem., 1996, 68, 291 CrossRef CAS; (c) G. Mehta and H. S. P. Rao, in Advances in Strain in Organic Chemistry, ed. B. Halton, JAI, London, 1997, vol. 6; Search PubMed; (d) G. Mehta and H. S. P. Rao, Tetrahedron, 1998, 53, 13325 CrossRef; (e) L. T. Scott, Pure Appl. Chem., 1999, 71, 209 CrossRef CAS.
- G. Mehta, S. R. Shah and K. Ravikumar, J. Chem. Soc., Chem. Commun., 1993, 1006 RSC; G. Mehta and K. Venkateswara Rao, Synlett, 1995, 319 CrossRef CAS; G. Mehta, K. Venkateswara Rao and K. Ravikumar, J. Chem. Soc., Perkin Trans. 1, 1995, 1787 RSC; G. Mehta, G. V. R. Sharma, M. A. Krishna Kumar, T. V. Vedavyasa and E. D. Jemmis, J. Chem. Soc., Perkin Trans. 1, 1995, 2529 RSC; G. Mehta, G. Panda, R. D. Yadav and K. Ravikumar, Indian J. Chem., Sect. B, 1997, 36, 301 Search PubMed; G. Mehta and G. Panda, Tetrahedron Lett., 1997, 38, 2145 CrossRef CAS; G. Mehta and G. Panda, Chem. Commun., 1997, 2081 RSC; G. Mehta, G. Panda, S. R. Shah and A. C. Kunwar, J. Chem. Soc., Perkin Trans. 1, 1997, 2269 RSC; G. Mehta, G. Panda and P. V. V. S. Sarma, Tetrahedron Lett., 1998, 39, 5835 CrossRef CAS.
- G. N. Sastry, E. D. Jemmis, G. Mehta and S. R. Shah, J. Chem. Soc., Perkin Trans. 2, 1993, 1867 RSC.
- W. H. Laarhoven, Th. J. H. M. Cuppen and R. J. F. Nivard, Tetrahedron, 1970, 26, 4865 CrossRef CAS.
- All new compounds reported here were fully characterised on the basis of their spectral (UV, IR, 2D 1H and 13C NMR, MS) and analytical data. Selected data for 3: mp 253 °C; λmax(MeOH)/nm 305, 275, 260 and 240; δH(300 MHz; CDCl3), 8.68 (2H, dd, J 6 and 3.3), 8.26 (2H, d, J 8.7), 7.95 (2H, d, J 8.7), 7.84 (4H, ABq, J 8.7), 7.76 (2H, dd, J 5.7 and 3.3); δC(75 MHz; CDCl3) 137.6 (qC), 135.4 (qC), 134.6 (qC), 133.1 (qC), 130.8 (qC), 130.5 (qC), 128.9 (qC), 127.5 (CH), 127.3 (CH), 127.1 (CH), 126.9 (CH), 125.1 (CH) and 124.3 (CH); m/z 300 (M+). For 4: mp >250 °C (decomp.); λmax(MeOH)/nm 319, 272, 257 (sh), 242 (sh); δH(300 MHz; CDCl3) 9.41 (2H, d, J 8.4), 8.83 (2H, d, J 7.5), 8.35 (2H, d J 8.7), 8.01 (2H, d, J 8.4), 7.91 (2H, s), 7.88–7.77 (4H, m); δC(75 MHz; CDCl3) 136.7 (qC), 134.2 (qC), 134.0 (qC), 133.9 (qC), 133.7 (qC), 130.2 (qC), 128.5 (qC), 127.8 (CH), 127.5 (CH), 127.1 (CH), 127.0 (CH), 126.5 (CH), 125.5 (CH), 124.5 (qC), 123.9 (CH); m/z 350 (M+). For 5: λmax(MeOH)/nm 347, 334, 279, 252; δH(400 MHz; CDCl3) 9.41 (2H, d, J 8), 8.86 (2H, d, J 7.2) 8.73 (2H, dd, J 6.4 and 3.6), 8.45 (4H, ABq, J 8.4), 7.87 (2H, d, J 8), 7.83 (2H, d, J 9.2), 7.79 (2H, dd, J 6 and 3.2); m/z 400 (M+)..
- Mono- 3 and tri-benzocorannulene 5 reported here have been prepared previously by Scott et al. [ref. 1(b), (e)] following entirely different routes. See also: B. McMahon, B.S. Thesis, Boston College, 1997; C. C. McComas, B.S. Thesis, Boston College, 1996. Since the details of this work are not published, we have provided here the spectral data and also compared the spectra of our synthetic compounds with theirs. Dibenzocorannulene 4 has been prepared for the first time..
- D. L. Nagel, R. Kupper, K. Antonson and L. Wallcave, J. Org. Chem., 1977, 42, 1977.
| This journal is © The Royal Society of Chemistry 2000 |