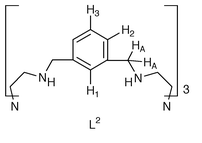
Steric constraint generating large through-space 1H–203,205Tl coupling in a dithallium(I) cryptate
Oliver W.
Howarth
a,
Jane
Nelson
b and
Vickie
McKee
c
aChemistry Department, University of Warwick, Coventry, UK CV4 7AL
bOpen University, Milton Keynes, UK MK7 6AA
cSchool of Chemistry, Queens University, Belfast, UK BT9 5AG. E-mail: jane.nelson@qub.ac.uk
First published on 7th January 2000
Abstract
An aminocryptand host enforces approach of encapsulated Tl(I) ions to just within 4.4 Å; despite this relatively large separation, strong Tl(I)⋯Tl(I) interaction is observed and the aromatic proton of the bridging link exhibits a large through-space coupling to the pair of equivalent Tl nuclei.
In recent years we have become interested in the consequences of enforced proximity between cations encapsulated within azacryptand hosts.1 In the transition series, this steric enforcement generates a previously uncharacterised bonding situation: a one-electron bond between copper ions in an average-valence +1.5 redox state.2–6 Steric enforcement of close approach for transition ions implies use of relatively small hosts such as (imBT)† and amBT(= imBT + 12H), while larger hosts such as (N[(CH2)2N
![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH(C6H4-
m)CH
CH(C6H4-
m)CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) N(CH2)2]3N
(L1) and its amino form L2 (= L1 + 12H)
have suitable cavity size to accommodate pairs of larger main group cations
such as Tl+. Our earlier studies with cryptates of
L27,8 and the analogous
iminocryptand ligand, L1, demonstrate9,10 that these hosts offer internuclear distances of
between 3 and 6 Å according to the preference of the guest cation, as
the cryptands can make use of a triple helical twist mechanism11,12 which allows them to adapt to the
preferred coordination site separation of the cationic guests.
N(CH2)2]3N
(L1) and its amino form L2 (= L1 + 12H)
have suitable cavity size to accommodate pairs of larger main group cations
such as Tl+. Our earlier studies with cryptates of
L27,8 and the analogous
iminocryptand ligand, L1, demonstrate9,10 that these hosts offer internuclear distances of
between 3 and 6 Å according to the preference of the guest cation, as
the cryptands can make use of a triple helical twist mechanism11,12 which allows them to adapt to the
preferred coordination site separation of the cationic guests.
There is interest13 among bonding theorists in interactions between the formally closed shell low-valent p-block cations. In a number of dimeric and oligomeric structures13,14 with Tl⋯Tl distances (some supported by bridging donors) ranging from 3.5 to 4.0 Å, there is evidence for varying degrees of s2⋯s2 interaction. Another point of interest is the possible existence of weakly attractive arene⋯heavy metal interactions,15, which could assist the close approach of Tl(I) ions.
The dithallium(I) cryptates of L2 are synthesised‡ by direct reaction of preformed ligand with the appropriate thallium(I) salt. 1H NMR studies on the triflate salt, T12L2(CF3SO3)21, in the solid state and in solution, suggest a simple and symmetric structure where each Tl(I) cation occupies the site defined by the N4 cap. There is evidence of a low-activation dynamic process in solution in that both methylene-cap signals of 1 present as broad temperature-independent singlet resonances with no discernible fine structure due to geminal or vicinal [1H,1H] methylene coupling. Sharp signals for the H2 and H3 resonances show well defined ortho coupling; however the expected singlet H1 resonance (Ar1), which in analogous L1 disilver(I) and dicopper(I) iminocryptates16 is strongly affected by guest encapsulation both in respect of breadth and position, appears here as a severely broadened triplet (apparent 1J (205,203Tl,1H) ≈ 17 Hz) at δ ca. 6.8. The triplet structure is just discernible at 400 MHz; broadening is a function of the magnetic field, approximating to 16∶9∶6 for 400, 300 and 250 MHz spectra, in proportion to the square of the magnetic field used [Fig 1(a)]. The signal from the methylene HA hydrogens adjacent to the aromatic ring is a similarly broadened triplet [Fig 1(b)] and decoupling experiments fail to relate the coupling of the broad triplets to any proton resonance. Also the resonances are not narrowed significantly at high temperature, thus ruling out broadening via an exchange process. These observations implicate through-space coupling to thallium, the latter relaxing by a chemical shift anisotropy mechanism.17 The splitting of the α-methylene signal confirms that the two 205,203Tl nuclei couple to each other with J(Tl,Tl) >>17 Hz, an example of the strong coupling case.17b The size of the coupling to the lone Ar1 hydrogen is unexpected because no formal bond to thallium exists. Nor can it be explained on a through-bond basis as, despite an identical bond pathway, the H3 resonance shows no thallium coupling. It appears that this coupling has to be explained on the basis of interaction between the lone aromatic hydrogen Ar1 and the pair of Tl(I) ions which, although unbonded, are constrained by geometry to lie in close proximity.
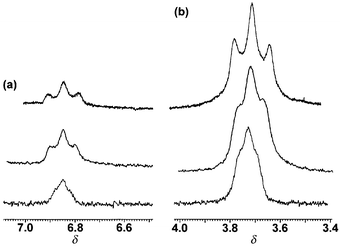 | ||
| Fig. 1 The Tl-coupled Ar1 (a) and CH2(A) (b) resonances in different magnetic fields: bottom trace, 400; middle trace, 300; top trace, 250 MHz. (Spectra run in CD3CN, 295 K, chemical shift in ppm from SiMe4). | ||
The low solubility of 1 in CD3CN allows only a weak 13C solution spectrum where the noise level hinders the unambiguous attribution of 205,203Tl, 13C couplings. There are indications of such coupling in the unique Ar1 carbon resonance which appears at δ ca.129.9 as a broadened ≈ 80 Hz triplet. The methylene carbons give rise to two sharp resonances at δ 57.5 and 49.3 with a broader, possibly Tl-coupled, feature at δ 54.1 representing the methylene carbon adjacent to the aromatic ring. In the CP MAS 13C spectrum signal breadth is generally sufficient to conceal any 205,203Tl,13C coupling although splitting of a weak resonance in the region δ 120–130 may derive from such a cause. The weak 15N CP MAS spectrum obtained for 1 likewise fails to show any coupling to thallium; just one relatively broad signal (half-width 150 Hz) centered at δ −307 (vs. NH4NO3 standard) appears, representing overlapped cryptand N(H) and Nbr resonances.
In order to confirm the hypothesis of close approach of Tl nuclei,
crystallographic evidence was sought,§
using the synchrotron source at Station 9.8 at CLRC Daresbury. The
structure is illustrated in Fig. 2. The
[Tl2L2]2+ cation has ![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) point
symmetry and the anion lies on a threefold axis, so the asymmetric unit
contains one sixth of the cation and one third of the anion. The Tl atom is
coordinated to the bridgehead nitrogen (N1) and to three secondary amine
groups (N11, N11A and N11B); it is displaced from the plane of the
secondary amines by 1.087(3) Å towards the centre of the cavity and
the Tl⋯TlA distance is 4.3755(4) Å. The Tl(I)
cations are thus farther from the Nbr apices than in other
dinuclear cryptates we have studied.1 The
Tl–N distances are around 2.7–2.8 Å, at the long end of
the range for Tl(I)–N distances,14,18,19 suggesting a predominantly ionic character for
bonding in this complex. The ligand host is fully extended to accommodate
the pair of large spherical cations, and there is no helicity of cryptand
strands. The structure is thus of unusually high symmetry for a cryptate.
The distance between Tl(I) cations and the Ar1
hydrogen is 3.816 Å. The shortest arene carbon⋯metal contact
is 4.207 Å , almost 0.5 Å longer than the van der Waals sum.
Edge to face intermolecular H⋯π contacts of ca. 2.49
Å involve all the arene rings.
point
symmetry and the anion lies on a threefold axis, so the asymmetric unit
contains one sixth of the cation and one third of the anion. The Tl atom is
coordinated to the bridgehead nitrogen (N1) and to three secondary amine
groups (N11, N11A and N11B); it is displaced from the plane of the
secondary amines by 1.087(3) Å towards the centre of the cavity and
the Tl⋯TlA distance is 4.3755(4) Å. The Tl(I)
cations are thus farther from the Nbr apices than in other
dinuclear cryptates we have studied.1 The
Tl–N distances are around 2.7–2.8 Å, at the long end of
the range for Tl(I)–N distances,14,18,19 suggesting a predominantly ionic character for
bonding in this complex. The ligand host is fully extended to accommodate
the pair of large spherical cations, and there is no helicity of cryptand
strands. The structure is thus of unusually high symmetry for a cryptate.
The distance between Tl(I) cations and the Ar1
hydrogen is 3.816 Å. The shortest arene carbon⋯metal contact
is 4.207 Å , almost 0.5 Å longer than the van der Waals sum.
Edge to face intermolecular H⋯π contacts of ca. 2.49
Å involve all the arene rings.
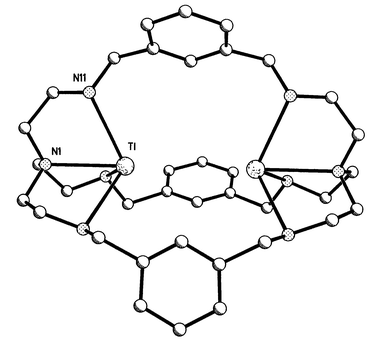 | ||
| Fig. 2 Structure of Tl2L2(CF3SO3)2. Selected distances (Å): Tl–N(I) 2.728(5), Tl–N(11) 2.796(3), Tl···Tl 4.3755(4), Tl⋯H(9) 3.816. | ||
The structural data make it clear that the sizeable coupling between the Ar1 proton and 205,203Tl is formally through space, although the average separation of these nuclei in the dynamic solution environment may not be identical with that in the crystal where intermolecular H⋯π packing effects operate. The thallium–thallium coupling of >> 17 Hz must also be classified as a through space effect given the relatively large separation of Tl(I) cations, almost 1 Å greater than the internuclear distance (3.4 Å) in the element. We believe that this represents the largest recorded through-space coupling.
In most dimeric or quasi-dimeric structures,13,14,19 Tl⋯Tl distances, in the range 3.6–3.9 Å are significantly shorter than noted here and the closest Tl⋯arene carbon distance of 3.816 Å in this structure exceeds that typical of weakly interacting arene–heavy metal systems.19 Nonetheless, we have clear evidence of coupling between both the pair of Tl(I) cations and between this Tl(I)2 pair and the unique aromatic proton Ar1, despite the ionic character which explains the absence of 203,205Tl, 1H couplings elsewhere in the cryptate spectrum. It is intriguing that the only 203,205Tl, 1H couplings observed in this cryptate are mediated via a non-bonded pathway, e.g. that involving steric compression of the Tl+⋯[H]⋯Tl+ moiety. The implication is that coupling information can be efficiently transmitted in large soft cations like Tl(I) by non-directional overlap of electron density.
Acknowledgements
We thank EPSRC for access to the Solid State NMR service at Durham and to SRS station 9.8 at Daresbury. We are indebted to Dr M. Arthurs of Coventry University for the 250 MHz 1 H NMR spectrum.References
- J. Nelson, V. McKee and G. Morgan, Prog. Inorg. Chem., 1998, 47, 167 Search PubMed.
- C. Harding, V. McKee and J. Nelson, J. Am. Chem. Soc., 1991, 113, 9684 CrossRef CAS.
- C. J. Harding, J. Nelson, M. C. R. Symons and J. Wyatt, J. Chem. Soc., Dalton Trans., 1994, 2499 Search PubMed.
- J. A. Farrar, V. McKee, A. H. R. al Obaidi, J. J. McGarvey, J. Nelson and A. J. Thomson, Inorg. Chem., 1995, 34, 1302 CrossRef CAS.
- J. A. Farrar, R. Grinter, F. Neese, J. Nelson and A. J. Thomson, J. Chem. Soc., Dalton Trans., 1997, 4083 RSC.
- A. H. R. al-Obaidi, G. Baranovich, J. L. Coyle, C. Coates, J. J. McGarvey, V. McKee and J. Nelson, Inorg. Chem., 1998, 37, 3597 CrossRef CAS.
- C. J. Harding, F. Mabbs, E. MacInnes, V. McKee and J. Nelson, J. Chem. Soc., Dalton Trans., 1996, 3227 RSC.
- A. Escuer, C. J. Harding, Y. Dussart, J. Nelson, V. McKee and R. Vicente, J. Chem. Soc., Dalton Trans., 1999, 223 RSC.
- C. J. Harding, Q. Lu, J. F. Malone, D. J. Marrs, N. Martin, V. McKee and J. Nelson, J. Chem. Soc., Dalton Trans., 1995, 1739 RSC.
- R. Abidi, M. G. B. Drew, F. Arnaud-Neu, S. Lahely, M. J. Schwing-Weil, D. J. Marrs and J. Nelson, J. Chem. Soc., Perkin Trans., 2, 1996, 2747 RSC.
- O. W. Howarth, G. G. Morgan, V. McKee and J. Nelson, J. Chem. Soc., Dalton Trans., 1999, 2097 RSC.
- M. G. B. Drew, D. Farrell, G. G. Morgan, V. McKee and J. Nelson, to be published..
- P. Pyykko, Chem. Rev., 1997, 97, 597 CrossRef.
- H. Schuman, C. Janiak, J. Pickardt and U. Nborner, Angew. Chem., Int. Ed. Engl., 1987, 26, 789 CrossRef; P. Jutzi, J. Schnittger and M. B. Hursthouse, Chem. Ber., 1991, 124, 1693 CAS; and references therein..
- C. Janiak, Coord. Chem. Rev., 1997, 163, 107 CrossRef CAS; H. Schuman, C. Janiak, M. A. Khan and J. J. Zuckermann, J. Organomet. Chem., 1988, 354, 7 CrossRef.
- Q. Lu, J.-M. Latour, C. J. Harding, N. Martin, D. J. Marrs, V. McKee and J. Nelson, J. Chem. Soc., Dalton Trans., 1994, 1471 RSC.
- (a) P. Ghosh, P. J. Desrosiers and G. Parkin, J. Am. Chem. Soc., 1998, 120, 10416 CrossRef CAS; (b) R. Freeman, Spin Choreography, Spektrum, Oxford, 1997, ch. 1, p. 29. Search PubMed.
- A. J. Amoroso, J. C. Jeffrey, P. L. Jones, J. A. McCleverty, E. Psillakis and M. D. Ward, J. Chem. Soc., Chem. Commun., 1995, 1175 RSC; K. Hellmann, L. H. Gade, R. Fleischer and T. Kottke, Chem. Eur. J., 1997, 3, 1801 CAS; and references therein..
- C. H. Galka and L. H. Gade, Inorg. Chem., 1999, 38, 1038 CrossRef CAS.
- G. M. Sheldrick, SHELX-97, University of Göttingen, 1997..
Footnotes |
† imBT =
N[(CH2)2N![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CHCH CHCH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) N(CH2)2
]3N. N(CH2)2
]3N. |
| ‡ Tl2 L2(CF3SO3)22H2O ·MeOH, 1: To 0.5 mmol L2 dissolved in 10 cm3 MeOH was added 1 mmol Tl(OAc) dissolved in 2 ml water followed by 1 mmol of LiCF3SO3 as solid. A colourless precipitate was obtained in ca. 70% yield on concentrating the pale yellow solution. The final crop of this preparation yielded the small single crystals used for crystallography. Anal. Found (calc.): C, 33.8(34.2); H, 3.9(4.4); N 8.1(8.2)%. |
| § Crystal data: [Tl2L2](CF3SO3)2, C38H54F6N8O6S2 Tl2, colourless needle, 0.16 × 0.06 × 0.04 mm, hexagonal, a = 9.5074(2), c = 29.7005(4) Å, U = 2324.97(8) Å3, space group P63/m, Z = 2, μ = 7.088 mm−1, F(000) = 1268. Data were collected at 150(2) K using a SMART CCD with synchrotron radiation (λ = 0.6885 Å, SRS station 9.8 at Daresbury). A hemisphere of data (16580 reflections, θmax = 29.33°) was collected. The structure was solved by direct methods and refined on F2, using all 2277 independent reflections (Rint = 0.0346). Non-hydrogen atoms were refined anisotropically and hydrogen atoms were inserted at calculated positions except for the amine proton, which was located and refined with a fixed ADP. The refinement, on 100 parameters, converged with wR2 = 0.0835, GOF = 1.060 (all data) and conventional R1 = 0.0357 (2ς data). The only significant residual peaks were close to the Tl atom. All programs used in the structure refinement are contained in the SHELX-97 package.20 CCDC 182/1483. See http://www.rsc.org/suppdata/cc/a9/a908476b/ for crystallographic files in .cif format. |
| This journal is © The Royal Society of Chemistry 2000 |