Hf27Si6P10, a novel metal-rich compound with P2 groups
Chang-Cang Huang, Jörg Neuhausen and Wolfgang Tremel*
Institut für Anorganische Chemie und Analytische
Chemie, Universität Mainz, Duesbergweg 10-14, D-55099, Mainz, Germany.. E-mail: tremel@indigotrem1.chemie.uni-mainz.de
First published on UnassignedUnassigned6th January 2000
Abstract
The new ternary metal rich compound Hf27Si6P10 has been synthesized by reduction of HfP with Hf and Si; Hf27Si6P10 crystallizes in a new structure type, a characteristic and unexpected feature of which is the presence of P2 groups; the structural results are interpreted with the aid of high-level band structure calculations.
The large family of metal-rich binary compounds that are formed between a transition metal (M) and a variety of main-group non-metals, metalloids and adjacent metals (Q) exhibit a remarkable range of compositions, structures and properties. It is surprising that these compounds challenge our basic assumptions about the chemistry of the transition elements and our chemical understanding in general. The existence and structure of none of these compounds can be predicted a priori and a rationalization of their structures a posteriori does not give chemically conclusive hints why seemingly simply binary compounds of the early transition metals (e.g. with composition M2Q) display as many differences as they do.1–5 Furthermore, the possibility of metal segregation in mixed-metal systems allows the formation of pseudo-binaries (M–M′)–Q which have no counterparts in the binary systems.6
Nonetheless, this group of compounds also shows some common characteristics regarding their structures as well as their electronic and bonding features. (i) In all known representatives the non-metal atoms are situated in capped trigonal-prismatic voids of the metal sublattice, and there are no filled voids adjacent to each other, i.e. from a topological point of view, there is no possibility for Q–Q bonding. (ii) Owing to appreciable differences in the electronegativities and valence energies of the component atoms, pnictides may be viewed as polar intermetallics; the more electronegative main-group element in these compounds contributes mostly to the low-lying valence band, especially for compounds of the early transition metals, whereas the conduction band has large contributions from the metal atoms. The stability is mostly based on strong heteroatom M–Q bonding; in addition, metal-rich phases also exhibit strong M–M but no Q–Q bonding.7
In the quest for new metal-rich quasi-binary early transition metal pnictides we have investigated reactions in the system M–Q–Q′ (M = Zr, Hf; Q = P, As, S, Se; Q′ = Si), the rationale behind this synthetic approach being to mimic the electron count in known binary compounds by the use of three components. Hf27Si6P10 was prepared in a two step synthesis. To avoid a loss of P during the arc melting, HfP8 was synthesised in a silica tube at 800 °C from the elements in a 1∶1 ratio. In the second step, stoichiometric mixtures of HfP, Hf and Si were pressed into pellets with a hydraulic press. The pellets were melted under argon on a water-cooled copper hearth. Reactions with the approximate starting compositions ‘Hf6SiP2’ led to homogeneous products. EDX investigations on selected crystals indicated a phase width of ±10% within the limits of accuracy. According to the results of the X-ray structure determination9 Hf27Si6P10 crystallizes in a new structure type which contains fragments of a bcc packing. The crystal structure is depicted in a projection along the short a axis in Fig. 1. All atoms are located at x = 0 or 0.5 along the crystallographic a axis, i.e. each layer in Fig. 1 is fused to identical layers above and below the projection plane. The unit cell contains 54 metal atoms (nine crystallographically independent metal atoms per asymmetric unit) and 32 Si and P atoms (which cannot be distinguished by X-ray diffraction). The P and Si atoms are located in mono-, bi- and tri-capped trigonal Hf prisms with Hf–P/Si bond lengths ranging between 2.572(6) and 3.083(7) Å, where the larger Si atoms are assumed to be located in the nine-coordinate sites (Si and P mixing on the non-metal positions, however, cannot be ruled out). Based on this assumption the composition Hf27Si6P10, in agreement with the analytical results, has been assigned. In an alternative description the Hf27Q16 structure may be viewed as being built up from M6Q8 clusters and cluster fragments.10 The Hf27Q16 structure contains two variants of fused M6Q8 clusters that are condensed via common corners along [100]: Hf5 fragments of the Hf6Q8 cluster where a Hf atom from the equatorial plane of the complete Hf6X8 unit is missing, and two types of Hf6Q8 clusters which are linked via common corners with Hf5 and Hf6 units. The first type of Hf6Q8 cluster (dark grey in Fig. 1) is linked to four Hf5 units (medium grey) in the projection plane to give an entity with a ‘bow-tie’ shape. The second type of Hf6Q8 cluster (shown in light grey) is connected to two Hf5 and two Hf6 units in such a way that four type-2 Hf6Q8 clusters form a tetrameric entity. The entity in turn is linked by sharing common corners with four Hf5 units. These condensed cluster units form a ribbon-like pattern along [001] which is highlighted in Fig. 1. Each of these ribbons is fused with two adjacent ribbons by Hf–Hf bonds and by sharing common P/Si atoms. Thus, all Hf atoms belong either to Hf5 or Hf6 units.
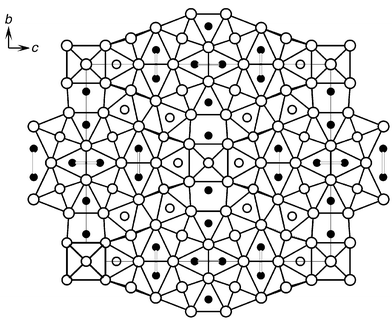 | ||
| Fig. 1 View of the Hf27Si6P10 structure along a. White circles, Hf atoms; grey circles, Si atoms; black circles, P atoms. | ||
The shortest Hf–Hf bonds occur between the apical and basal atoms of the cluster units [distances ranging from 3.035(1) to 3.215(1) Å], whereas the equatorial Hf–Hf distances are significantly longer [3.544(2)–3.825(2) Å]. As is apparent from the length of the b axis, the octahedra are tetragonally compressed; as a result, the distances between the apical Hf atoms are 3.573(1) Å.
The most important and unexpected feature of the Hf27Q16 structure are two short P–P separations of 2.58(2) and 2.69(13) Å which are clearly in the bonding range. In addition, the Hf–Hf contacts across the P–P units are, at 3.270(2) and 3.225(1) Å, very short. Based on Pauling’s formula11dn = d1 − 0.6 log n, the bond orders for the P–P contacts are 0.23 and 0.15 i.e. P–P bonding is present to a significant extent. These empirical results are also supported by the results of first-principles ab initio band structure calculations (vide infra) that have been performed on hypothetical Hf27P16 (the binary electron-rich end member of the ternary system Hf27SixP16−x where the weakest P–P interactions might be expected). The presence of non-metal–non-metal bonding interactions in metal-rich compounds of an early transition metal is remarkable for at least two reasons: (i) since the non-metal atoms are the more electronegative partners in these compounds, the non-metal states should be occupied whereas the high-lying metal states are about to be filled. As a result, bonding interactions between non-metal atoms are unlikely. (ii) The non-metal atoms are situated in the voids of the metal sublattice and the probability of having filled voids adjacent to each other decreases with increasing M∶Q ratio.
The Hf27Q16 structure contains two adjacent bicapped trigonal-prismatic sites. One of them is embranced by four Hf6 clusters of the tetrameric unit, while the second is located at the intersection of the tetrameric unit and the ‘bow-tie’ shaped fragment. In order to clarify if this topological arrangement is only accidental, or if P–P interactions are significant for the structure stability, we have analysed the electronic structure of the title compound.
Considering the short P–P contacts as P–P bonds the electronic structure of Hf27Q16 may be formally described as (Hf1.78+)27(Si4−)6(P 24−)3(P3−)4 , i.e. approximately two electrons are available for each Hf atom for metal–metal bonding. According to the results of TB–LMTO–ASA calculations12 the 3p block of the Si and P atoms is located well below the Fermi level. Fig. 2 shows the computed total density of states for hypothetical ‘Hf27P16’ with the metal and non-metal contribution [Fig. 2(a)] as well as the results of the P–P bonding analysis in form of the COHP plot [Fig. 2(b)]. The P–P bonding states are separated by a small gap from the antibonding states that appear to be very localised just below −2 eV. The strong localisation indicates that a fraction of the P 3p orbitals is preferentially engaged in P–P bonding. A significant density of states is found at the Fermi level. Considering the M–M bonds along all directions in the structure of Hf27Q16, metallic properties can be safely assumed. The different site preferences for Si and P can be understood based on the differences in M–Q bonding. The bonding analysis of ‘Hf27P16’ shows that higher bond orders are associated with the higher coordinated sites, and this situation should be increased for Si because of the greater expansion of the 3p orbitals and the lower electronegativity of Si relative to P.
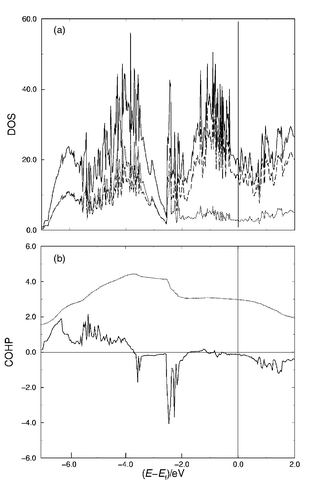 | ||
| Fig. 2 (a) LMTO density of states for ‘Hf27P16’; solid line: total DOS; dashed line: Hf contribution; dotted line: Si/P contribution. The Fermi level is indicated by the vertical bar. (b) Crystal orbital Hamiltonian populations (COHP) for the P–P interactions. Upper part of the diagram is bonding, the lower part antibonding. The dotted line represents the integrated P–P overlap population. | ||
In summary, Hf27Q16 is a metal-rich compound of an early transition metal whose structure is stabilized to a significant extent by bonding between the non-metal atoms. This is associated with a partial electron transfer from the anionic to the cationic components, which has also been observed for metal tellurides;13,14 a comparable feature has been reported for two compounds of the heavy group homologues, Hf6TiSb415 and (Zr, V)13Sb10,16 but is unprecedented for the lighter congeners.
Acknowledgements
This work was supported by the Fonds der Chemischen Industrie. We are indebted to Heraeus Quarzschmelze Hanau (Dr Höfer) for a generous gift of silica tubes.References
- Ti2P: ; P. O. Snell, Acta Chem. Scand., 1968, 22, 1942 Search PubMed; Zr2P: ; P. J. Ahlsen, Z. Kristallogr., 1989, 189, 117 Search PubMed; Hf2P: ; T. Lundström, Acta Chem. Scand., 1968, 22, 1801 Search PubMed; Zr2As: ; S. Rundqvist, Acta Chem. Scand., 1968, 22, 2395 Search PubMed; Hf2As: ; J. O. Willerström, Acta Chem. Scand., Ser. A, 1984, 38, 91 Search PubMed.
- Ti2S: ; J. P. Owens and H. F. Franzen, Acta Crystallogr., 1967, 23, 77 Search PubMed; Zr2S: CrossRef CAS; B. R. Conard, High Temp. Sci., 1971, 3, 491 CrossRef CAS; Hf2S: ; H. F. Franzen, Z. Kristallogr., 1966, 123, 1331 Search PubMed; Zr2Se: ; H. F. Franzen, Acta Crystallogr., Sect. B, 1968, 24, 801 Search PubMed; Ti2Se, Hf2Se: ; H. F. Franzen, J. Smeggil and B. R. Conard, Mater. Res. Bull., 1967, 2, 1087 Search PubMed; Zr2Te: ; G. Örlygsson and B. Harbrecht, Inorg. Chem., 1999, 38, 3377 Search PubMed; Hf2Te: ; B. Harbrecht, M. Conrad, T. Degen and R. Herbertz, J. Alloys Compd., 1997, 255, 178 Search PubMed.
- Nb2P: ; Y. B. Kuzma, Dop. Akad. Nauk Ukr. RSR, Ser. B, 1988, 121, 47 Search PubMed; Ta2P: ; A. Nylund, Acta Chem. Scand., 1966, 20, 2393 Search PubMed; Ta2As: ; S. Rundqvist, Acta Chem. Scand., 1969, 23, 2188 Search PubMed.
- Ta2S: ; H. F. Franzen and J. Smeggil, Acta Crystallogr., Sect. B, 1969, 25, 1736 Search PubMed; Ta2Se: CrossRef CAS; B. Harbrecht, Angew. Chem., Int. Ed. Engl., 1989, 28, 1660 CrossRef CAS.
- Sc2Te: ; P. A. Maggard and J. D. Corbett, Angew. Chem., Int. Ed. Engl., 1997, 36, 1974 Search PubMed.
- Nb1.72Ta3.28S2: ; X. Yao and H. F. Franzen, J. Am. Chem. Soc., 1991, 113, 1426 Search PubMed; Zr6.45Nb4.55P4: Chem. Mater., 1993, 5, 678; CrossRef CAS; Hf5.08Mo0.92P3: CrossRef CAS; J. Cheng and H. F. Franzen, J. Solid State Chem., 1996, 121, 362 CrossRef CAS.
- E. Garcia and J. D. Corbett, Inorg. Chem., 1988, 27, 2353 CrossRef CAS.
- W. Jeitschko and H. Nowotny, Monatsh. Chem., 1962, 93, 1284 CAS.
- Crystal data (Bruker, CCD, T = 298 K) for Hf27Si6P10: M = 1317, orthorhombic, space group Immm; a = 3.5739(7), b = 18.623(4), c = 23.081(5) Å, Z = 2, λ = 0.71073 Å, Dc = 11.443 g cm−3, μ(Mo-Kα) = 91.46 mm−1, 1029 independent reflections, unique data with I > 2σ(I), 809, R(F) = 0.035, Rw(F2) = 0.098, number of variables, 75. Structure solved and refined using the SHELXTL-Plus program system. Crystal needle-like, dimensions 0.005 × 0.005 × 0.1 mm. An empirical absorption correction was applied to the data (min. max. transmission, 0.080, 0.531). CCDC 182/1426. See http://www.rsc.org/suppdata/cc/a9/a906873b/ for crystallographic files in .cif format..
- A. Simon, Angew. Chem., Int. Ed. Engl., 1981, 20, 1 CrossRef.
- L. Pauling, The Nature of the Chemical Bond, Cornell University Press, Ithaca, New York3rd edn., 1948. Search PubMed.
- U. van Barth and L. Hedin, J. Phys. C, 1971, 4, 2064 Search PubMed; O. K. Andersen, Phys. Rev. B: Condens. Mather, 1975, 12, 3060 Search PubMed; H. L. Skriver, The LMTO Method, Springer, Berlin, 1984; Search PubMed; O. Jepsen and O. K. Andersen, Solid State Commun., 1971, 9 Search PubMed.
- J. Rouxel, Chem. Eur. J., 1996, 2, 1053 CAS.
- Nb2Te3: ; H. Kleinke and W. Tremel, Chem. Commun., 1999, 1175 Search PubMed; Ta4BTe8: RSC; H. Kleinke, E. W. Finckh and W. Tremel, Angew. Chem., Int. Ed., 1999, 38, 2054 RSC.
- H. Kleinke, Inorg. Chem., 1999, 38, 2931 CrossRef CAS.
- H. Kleinke, Chem. Commun., 1998, 2219 RSC.
| This journal is © The Royal Society of Chemistry 2000 |
