End-group modification of regioregular poly(3-alkylthiophene)s
Bea M. W. Langeveld-Voss, René A. J. Janssen*, A. J. H. Spiering, Joost L. J. van Dongen, Erik C. Vonk and Henk A. Claessens
Laboratory of Macromolecular and Organic Chemistry and Laboratory
of Instrumental Analysis, Eindhoven University of Technology, PO Box 513, 5600 MB, Eindhoven, The Netherlands.. E-mail: r.a.j.janssen@tue.nl
First published on UnassignedUnassigned7th January 2000
Abstract
An efficient and simple procedure for end-group modification of poly(3-alkylthiophene)s is presented which can be incorporated into the last step of the polymerisation reaction or employed as a post-polymerisation modification.
Poly(3-alkylthiophene)s (P3ATs) constitute an important class of conjugated polymers.1 Being chemically and thermally stable materials, soluble P3ATs are attractive for exploring their electronic and optical properties. The side chains not only ensure solubility, but are a key parameter for the mesoscopic structure in thin films and, hence, as a property determining element. Perfection of the primary structure of the polymer, including molecular weight and polydispersity, is a prerequisite to obtain well-defined materials in a reproducible manner. Recently, regioregular P3ATs have been incorporated as the active material in transistors in which a preferential supramolecular ordering of the polymer chains induces a high field-effect mobility of up to 0.1 cm2 V−1 s−1,2,3 approaching that of single crystal oligothiophenes.4 End-group functionalisation of these polymers would open the possibility to graft P3ATs onto surfaces, prepare block copolymers, or attach specific electroactive end groups and thereby extend the range of applications of these materials. Here we describe a simple and convenient procedure to introduce new end groups on the P3ATs.
Various synthetic routes to obtain regioregular P3ATs have been advanced over the last decade.5-8 The McCullough route is most frequently employed to prepare regioregular P3ATs (Scheme 1). Typically a 3-alkyl-2-bromothiophene is converted to the (4-alkyl-5-bromo-2-thienyl)magnesium bromide Grignard reagent, which is polymerised in situ using Ni(dppp)Cl2 as catalyst.5 The work-up procedure involves extensive Soxhlet extractions with MeOH, hexanes, CH2Cl2 and CHCl3,9 and provides fractionated polymers with a polydispersity (PD) = 1.3–1.5 and molecular weight (Mw) increasing from 4–5 kg mol−1 for the hexanes fraction to ≡30 kg mol−1 for the CHCl3 fraction as determined by SEC analysis in CHCl3 against polystyrene standards. Although regioregularities exceeding 99% have been claimed previously for P3ATs synthesised in this way, it has become clear that these polymers are less perfect than presumed initially.10,11 Elemental analysis has revealed that synthesised P3ATs (where the alkyl group is n-butyl, n-hexyl, n-octyl or n-decyl) may contain a considerable amount of bromine (typically 1–2 wt%).10,11†
 | ||
| Scheme 1 | ||
By investigating the hexane Soxhlet fraction of poly(3-hexylthiophene) (P3HT) with HPLC and preparative SEC in combination with MALDI-TOF mass spectrometry, it became clear that polymer chains terminated with 0, 1 and 2 bromines are present (Fig. 1).10,11‡ HPLC analysis of the hexane fraction confirms the presence of at least two series of P3HTs with alternating signal intensities [Fig. 2(a)]. The main progression in the HPLC trace [Fig. 2(a)] is identified as regioregular P3HT containing one bromine end group. This result is corroborated by the 1H NMR spectrum of the CHCl3 fraction of these polymers, which typically exhibits peaks for the benzylic resonances at δ 2.80 and 2.60 in a 20∶1 ratio. While the δ 2.80 signal is due to benzylic protons in a regioregular head-to-tail dyad, the signal at δ 2.60 can be assigned to regioirregular head-to-head dyads and bromine terminated thiophene end groups.9,12 The ratio of the benzylic 1H NMR signals is a more sensitive gauge6 to determine the regularity of the polymer than the aromatic protons and provides values on the order of 95% for the best materials.
 | ||
| Fig. 1 MALDI-TOF of a narrow molecular weight fraction (Mw = 2.96 kg mol−1, PD = 1.05) of P3HT obtained by preparative SEC showing the signals of chains with no, one and two bromine end groups. Note that SEC calibrated against polystyrene standards overestimates the molecular weight by a factor of 1.6. | ||
 | ||
| Fig. 2 HPLC of P3HT hexanes fraction before (a) and after (b) introduction of thiophene end groups. The numbers in the top graph indicate the number of thiophene rings of P3HT oligomers carrying one bromine end group. | ||
Although the terminal bromines constitute a structural and chemical imperfection, they can be utilised to modify the polymers by introducing new end groups. We found that a post-polymerisation reaction of the polymer with a thiophene Grignard reagent under addition of fresh catalyst works rather well.§ Analysis with HPLC of a sample treated in this way showed essentially a single progression of P3HT chains [Fig. 2(b)]. To get a greater insight into the efficiency of the end-group modification reaction, a sample of P3HT was treated with 5-trimethylsilyl-2-thienylmagnesium bromide to introduce 5-trimethylsilyl-2-thienyl end groups under addition of fresh catalyst. MALDI-TOF analysis of short chains gives unambiguous evidence of the incorporation of the (5-trimethylsilyl-2-thienyl) end groups, with no signals for bromine-terminated chains (Fig. 3). The MALDI-TOF spectrum shows that both α and α,ω substitution occurs. The introduction of trimethylsilyl groups is also supported by the 1H NMR spectrum, which shows a signal at δ 0.33.
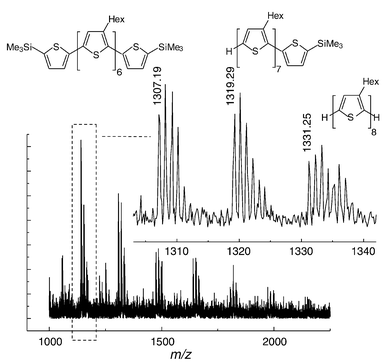 | ||
| Fig. 3 MALDI-TOF of P3HT after end-capping with 5-trimethylsilyl-2-thienyl groups. | ||
In addition to adding a Grignard reagent at the end of the polymerisation reaction together with a fresh amount of catalyst to introduce specific end groups, the same reaction can also be performed on previously synthesised and isolated material.¶ This post-polymerisation reaction leaves the polymer intact, as inferred from a comparison of the molecular weights before and after performing the end-capping reaction.
In conclusion we have shown that via the Ni(dppp)Cl2 mediated Grignard coupling it is possible to modify the bromine end groups that result from a McCullough type polymerization reaction of P3ATs, either as the last step in the polymerization or in a post-synthesis procedure on already isolated material. The procedure can be used to introduce end groups that are reactive to surfaces or to other functional groups. This opens the way to use P3ATs in adhesion, block-copolymerisation, electrooptical end groups and energy harvesting.
Acknowledgements
Financial support from the European Commission (Esprit 24793) is gratefully acknowledged.References
- J. Roncali, Chem. Rev., 1992, 92, 711 CrossRef CAS; Handbook of Organic Conductive Molecules and Polymers, ed. H. S. Nalwa, Wiley, New York, 1997, vol. 1–4; Search PubMed; Polythiophenes: Electrically Conductive Polymers, ed. G. Schopf and G. Koβmehl, Springer, Berlin, 1997; Search PubMed; Handbook of Conducting Polymers, 2nd edn., ed. T. A. Skotheim, R. L. Elsenbaumer and J. R. Reynolds, Marcel Dekker, New York, 1998. Search PubMed.
- H. Sirringhaus, P. J. Brown, R. H. Friend, M. M. Nielsen, K. Bechgaard, B. M. W. Langeveld-Voss, A. J. H. Spiering, R. A. J. Janssen, E. W. Meijer, P. Herwig and D. M. de Leeuw, Nature,, 1999, 401, 685 CrossRef CAS.
- Z. Bao, A. Dodabalapur and A. Lovinger, Appl. Phys. Lett., 1996, 69, 4108 CrossRef CAS.
- G. Horowitz, F. Garnier, A. Yassar, R. Hajlaoui and F. Kouki, Adv. Mater., 1996, 8, 52 CAS; J. H. Schön, C. Kloc, R. A. Laudise and B. Batlogg, Phys. Rev. B, 1998, 58, 12952 CrossRef CAS.
- R. D. McCullough and R. D. Lowe, J. Chem. Soc., Chem. Commun., 1992, 70 RSC; R. D. McCullough, R. D. Lowe, M. Jayaraman and D. L. Anderson, J. Org. Chem., 1993, 59, 904 CrossRef.
- T.-A. Chen, X. Wu and R. D. Rieke, J. Am. Chem. Soc., 1995, 117, 233 CrossRef CAS.
- S. Guillerez and G. Bidan, Synth. Met., 1998, 93, 123 CrossRef CAS.
- A. Bolognesi, W. Porzio, G. Bajo, G. Zannoni and L. Fannig, Acta Polym., 1999, 50, 151 CrossRef CAS; R. S. Loewe, S. M. Khersonsky and R. D. McCullough, Adv. Mater., 1999, 11, 251 CrossRef CAS.
- M. Trznadel, A. Pron, M. Zagorska, R. Chrzaszcz and J. Pielichowski, Macromolecules, 1998, 31, 5051 CrossRef CAS.
- B. M. W. Langeveld-Voss, Thesis, Eindhoven University of Technology, ISBN 90-386-0668-0, 1998..
- J. Liu, R. S. Loewe and R. D. McCullough, Macromolecules, 1999, 32, 5777 CrossRef CAS.
- G. Bidan, A. De Nicola, V. Enée and S. Guillerez, Chem. Mater., 1998, 10, 1052 CrossRef CAS.
Footnotes |
| † The bromine content was determined by Schöniger combustion. |
| ‡ The molecular mass of the polymers was measured using a Perseptive DE PRO Voyager MALDI-TOF spectrometer utilising a α-cyano-4-hydroxycinnamic acid matrix. A solution of 1 mg ml−1 P3AT in THF was mixed with the matrix material at a molar ratio of ca. 1∶10–3 (matrix∶P3AT) and crystallised on the target immediately before measurement. HPLC column specifications: Waters, Nova-Pak C18, 60 Å, 4 μm, dimensions 3.9 × 150 mm. The HPLC runs (detected at 400 nm) were performed with an MeCN–THF gradient of 90∶10 to 20∶80, 1% min−1, with Supra Gradient solvents from Biosolve. |
| § In a typical procedure distilled Pri2NH (2.8 ml, 20 mmol) was dissolved in 70 ml of dry THF. At room temperature BuLi (10.6 ml, 1.6 M in hexane, 17 mmol) was added. After stirring for 1.5 h, the solution was cooled to −60 °C and 2-bromo-3-hexylthiophene (4.0 g, 16 mmol) was added. The mixture was stirred for 1 h at –60 °C, and for 1 h at −40 °C. At −65 °C MgBr2·OEt2 (4.4 g, 17 mmol) was added. The mixture was allowed to warm to 0 °C slowly. When all MgBr2·OEt2 was dissolved, 0.09 g (0.18 mmol) Ni(dppp)Cl2 was added and the mixture was stirred at room temperature for 20 hs. Then 20 mmol of freshly prepared 2-thienylmagnesium bromide (X = H) or 5-trimethylsilyl-2-thienylmagnesium bromide [X = Si(CH3)3] in Et2O were added together with another portion of 0.18 mmol of Ni(dppp)Cl2 and stirring was continued for 20 h. The reaction mixture was precipitated in MeOH, and further purified via Soxhlet extractions using MeOH, hexane and CH2Cl2. Isolation via Soxhlet extraction with CHCl3 gave Mw = 25.6 kg mol−1 (PD = 1.42) for X = H and Mw = 14.6 kg mol−1 (PD = 1.48) for X = Si(CH3)3. |
| ¶ To a mixture of P3HT (0.50 g, Mw = 11.0 kg mol−1, PD = 1.4) in 15 ml THF and Ni(dppp)Cl2 (0.4 g, 0.7 mmol) was added freshly prepared 2-thienyl magnesium bromide (5.9 g, 36 mmol 2-bromothiophene and 0.9 g, 37 mmol Mg) in 15 ml THF. The mixture was refluxed for 3 h. After removal of THF the P3HT was fractionated by Soxhlet extractions with MeOH, CH2Cl2 (0.32 g, Mw = 10.8 kg mol, PD = 1.51) and CHCl3 (0.15 g, Mw = 19.4 kg mol−1, PD = 1.23), respectively. The same work-up procedure on the original P3HT sample afforded Mw = 12.0 kg mol−1 (PD = 1.27) and Mw = 19.9 kg mol−1 (PD = 1.23). |
| This journal is © The Royal Society of Chemistry 2000 |
