First Dewar benzene approach to acetylenic oligophenylene macrocycles: synthesis and structure of a molecular rectangle bearing two spindles
Masakazu
Ohkita
,
Kohta
Ando
,
Ken-ichi
Yamamoto
,
Takanori
Suzuki
and
Takashi
Tsuji
*
Division of Chemistry, Graduate School of Science, Hokkaido University, Sapporo, 060-0810, Japan.. E-mail: tsuji@sci.hokudai.ac.jp
First published on 7th January 2000
Abstract
A novel Dewar benzene approach to the construction of oligophenylene macrocycles has been developed by introducing Dewar benzene 1 as a building block and applied to the synthesis of a molecular rectangle 8, whose structure was characterized crystallographically.
During the course of our study on small cyclophanes generated from the corresponding Dewar derivatives,1 we were interested in exploiting a Dewar benzene for the construction of macrocyclic structures, especially for the synthesis of p-phenylene-based rigid, and thus strained, macrocycles. Since the bent shape of Dewar benzene is expected to provide a unique template effect on the cyclization, novel macrocyclic systems which are difficult to access from planar benzene derivatives may well become available based on the Dewar benzene approach. Here we report the rational and efficient synthesis of a novel rectangular macrocycle 8 using acetal-bridged Dewar benzene 1 as a building block, together with its crystallographic characterization. Phenylene acetylene macrocycles have attracted considerable recent attention in supramolecular chemistry and materials science.2
Dewar benzene 1 was readily prepared in four steps and on a gram scale from DMAD and 1,2-dichloroethylene.3 Palladium-catalyzed coupling of 1 with 2† afforded diethynylation product 3 (55% yield), which was then deprotected to 4 in 80% yield (Scheme 1). Copper-mediated oxidative coupling of 4 with CuCl/Cu(OAc)24 in pyridine under pseudo-high-dilution conditions produced cyclic dimer 5‡ in 70% yield. Acid hydrolysis of 5 followed by the silylation of resulting tetraol 6 afforded 7§ in 73% yield. Photochemical isomerization of 7 proceeded smoothly and cleanly by irradiation with a high-pressure mercury lamp through Pyrex at 12 °C to afford 8§ in quantitative yield. The less symmetrical product resulting from the rearrangement of only one of the Dewar benzene units was not detected by 1H NMR monitoring of the reaction.
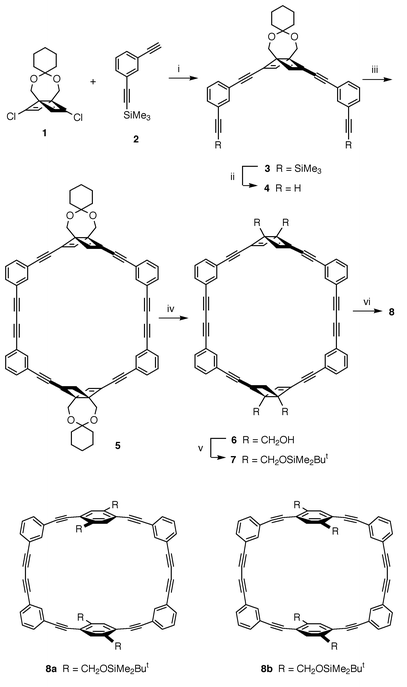 | ||
| Scheme 1 Reagents and conditions: i, Pd(PPh3)4, CuI, Et3N, 45 °C, 55%; ii, Bu4NF, THF, 0 °C, 80%; iii, CuCl, Cu(OAc)2, Py, syringe-pump addition over 2 h, 60 °C, 70%; iv, aq. HCl, THF, room temp., 98%; v, ButMe2SiOTf, Et3N, CH2Cl2, room temp., 75%; vi, hν, CH2Cl2, 12 °C, 100%. | ||
It is interesting to note that, in the 1H NMR spectra, the benzylic protons of 7 appear as a pair of AB doublets (δ 4.10 and 4.12, J = 11 Hz) while those of 8 appear as a singlet (δ 4.95), indicating that these protons are diastereotopic in 7 and operationally enantiotopic in 8. These observations would be most reasonably explained by postulating fast interconversion between the rotamers 8a and 8b, namely, fast rotation of the p-phenylene unit(s) in 8 on the NMR timescale despite the strained nature of the macrocycle.5
Crystals of 8 suitable for X-ray structure determination
(Fig. 1) were obtained by slow diffusion of
EtOH into a solution of 8 in CHCl3 at room
temperature.¶
The molecule adopts a nearly planar conformation in the crystal and the
dimension of framework is 7.32 × 11.54 Å, as defined by the
four inner m-phenylene carbon atoms. Interestingly, the framework
appears to be distorted to a parallelogram to accommodate two
SiMe2OCH2 moieties inside the cavity. The twist
angles of the benzene rings from the least-square plane formed by the
acetylenic carbon atoms are 3.7 and 0.4° in the m-phenylene
units and 3.4° in the p-phenylene units. The benzene rings are
essentially planar (deviation < 0.01 Å) while the acetylene units
show small deviation from linearity with the C![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) C–C angles of
168.7(3)–179.9(3)°.
C–C angles of
168.7(3)–179.9(3)°.
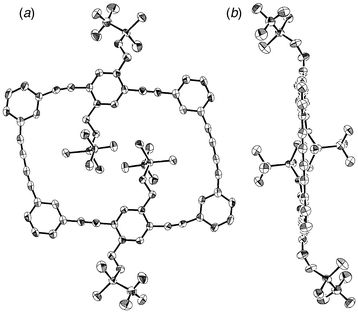 | ||
| Fig. 1 Molecular structure of 8: (a) top view, (b) side view. Hydrogen atoms are omitted for clarity. | ||
In conclusion, we have successfully synthesized and characterized a novel rectangular macrocycle 8 by introducing 1 as a building block. The high efficiency of the macrocyclization of 4 into 5 demonstrates the synthetic utility of a Dewar structure as a ‘masked’ p-phenylene unit. Further elaboration and application of the present Dewar benzene strategy are now in progress.
Acknowledgements
We thank Professor Tamotsu Inabe, Hokkaido University, for the use of X-ray analytical facilities. This work was supported in part by a Grant-in-Aid for Scientific Research (No. 09640620) from the Ministry of Education, Science, Sports and Culture of Japan.References
- T. Tsuji, Advances in Strained and Interesting Organic Molecules, ed. B. Halton, JAI Press, Greenwich, 1999, vol. 7, p. 103 and references cited therein. Search PubMed.
- Reviews: ; U. H. F. Bunz, Y. Rubin and Y. Tobe, Chem. Soc. Rev., 1999, 28, 107 Search PubMed; R. Faust, Angew. Chem., Int. Ed., 1998, 37, 2825 RSC.
- T. Tsuji, H. Watanabe, J. Tanaka, M. Inada and S. Nishida, Chem. Lett., 1990, 1193 CAS.
- A. de Meijere, S. Kozhushkov, T. Haumann, R. Boese, C. Puls, M. J. Cooney and L. T. Scott, Chem. Eur. J., 1995, 1, 124 CAS.
- Similar rotaion of spindles in strain-free phenylacetylene macrocycles, see: ; T. C. Bedard and J. S. Moore, J. Am. Chem. Soc., 1995, 117, 10 662 Search PubMed; S. Höger, A.-D. Meckenstock and S. Muller, Chem. Eur. J., 1998, 4, 2423 CrossRef CAS.
- T. X. Neenan and G. M. Whitesides, J. Org. Chem., 1988, 53, 2489 CrossRef CAS.
Footnotes |
| † Compound 2 was prepared by partial desilylation of 1,3-bis[(trimethylsilyl)ethynyl]benzene (ref. 6) with aqueous KOH/MeOH in 60% yield. |
| ‡ The isolated 5 was spectroscopically homogeneous although two stereoisomers arising from relative orientation of the Dewar units are possible. Only one of the possible isomers is depicted in Scheme 1. Compounds 6 and 7 were also spectroscopically homogeneous. |
| § Selected data for 7: δH(400 MHz, CD2Cl2) 0.08 (s, 24H), 0.92 (s, 36H), 4.10 (d, J 11.0, 4H), 4.12 (d, J 11.0, 4H), 6.83 (s, 4H), 7.29 (t, J 7.8, 4H), 7.38 (dt, J 7.8, 1.5, 4H), 7.52 (dt, J 7.8, 1.5, 2H), 7.70 (t, J 1.5, 2H); δC(100 MHz, CD2Cl2) −5.36, −5.23, 18.61, 26.05, 60.93, 66.42, 74.62, 81.31, 85.05, 94.77, 122.42, 124.11, 129.12, 131.75, 131.85, 136.18, 137.14, 146.02. For 8: δH(400 MHz, CD2Cl2) 0.15 (s, 24H), 0.96 (s, 36H), 4.95 (s, 8H), 7.36 (t, J 7.8, 4H), 7.41 (dt, J 7.8, 1.5, 4H), 7.49 (dt, J 7.8, 1.5, 2H), 7.66 (s, 4H), 7.88 (t, J 1.5, 2H); δC(100 MHz, CD2Cl2) −5.07, 18.82, 26.24, 63.56, 75.37, 82.73, 89.08, 95.11, 120.79, 122.62, 124.36, 129.32, 130.13, 130.34, 130.92, 139.73, 142.36. |
¶
Crystal data for 8:
C80H88O4Si4, M =
1224.6, colorless plate (0.60 × 0.30 × 0.05 mm), triclinic,
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 12.926(1), b = 17.570(2),
c = 8.074(1) Å, α = 93.356(4), β = 97.690(3),
γ = 79.755(4)°, V = 1787.1(3) Å3,
Dc = 1.14 g cm−3, Z
= 1, Mo-Kα radiation (λ = 0.71073 Å), T = 203
K, μ = 1.3 cm−1, F(000) = 656. A total of 7513
unique reflections (2θmax = 55°) were collected, of
which 5339 observed reflections [I > 3ς(I)]
were used in the structure solution (direct methods) and refinement
(full-matrix least-squares with 398 parameters) to give final R =
0.063 and wR = 0.069. Residual electron density is 0.45 e
Å−3. CCDC 182/1496. , a = 12.926(1), b = 17.570(2),
c = 8.074(1) Å, α = 93.356(4), β = 97.690(3),
γ = 79.755(4)°, V = 1787.1(3) Å3,
Dc = 1.14 g cm−3, Z
= 1, Mo-Kα radiation (λ = 0.71073 Å), T = 203
K, μ = 1.3 cm−1, F(000) = 656. A total of 7513
unique reflections (2θmax = 55°) were collected, of
which 5339 observed reflections [I > 3ς(I)]
were used in the structure solution (direct methods) and refinement
(full-matrix least-squares with 398 parameters) to give final R =
0.063 and wR = 0.069. Residual electron density is 0.45 e
Å−3. CCDC 182/1496. |
| This journal is © The Royal Society of Chemistry 2000 |
