Thermotropic and lyotropic behavior of novel amphiphilic liquid crystals having hydrophilic poly(ethyleneimine) units
Seiji
Ujiie
* and
Yumi
Yano
Department of Material Science, Interdisciplinary Faculty of Science and Engineering, Shimane University, Matsue, 690-8504, Japan
First published on 6th January 2000
Abstract
Novel ionic amphotropic liquid crystals, in which a hydrophilic poly(ethyleneimine) chain with hydroxy side-groups was attached to an azobenzene mesogenic core through an alkylene chain, showed a thermotropic smectic A mesophase and lyotropic smectic A and C mesophases.
Amphiphilic compounds such as ionic and nonionic surfactants always consist of hydrophilic and lipophilic groups. They can form thermotropic liquid crystalline phases in themselves and lyotropic liquid crystalline phases upon the addition of a solvent such as water.1 The thermotropic liquid crystalline systems of the amphiphiles build up layered structures with distinct arrangements of the molecules within their layers. In the lyotropic liquid crystalline systems, the hydrophilic group conveys water solubility, while the hydrophobic group drives the formation of self-assembled aggregates. Lyotropic liquid crystalline systems can form liquid crystalline phases such as lamellar mesophases, which resemble thermotropic smectic A phases, columnar and cubic mesophases. Surfactants with an aromatic mesogenic group can show liquid crystal formation due to the anisotropy formed by aggregation of the polar head groups and interactions between the aromatic mesogenic groups.2–4 They can show both thermotropic and lyotropic liquid crystalline behavior. It is of high importance in the field of supramolecular chemistry that new thermotropic and lyotropic liquid crystal forming amphiphilic compounds are sought. This makes it possible to develop novel types of functional liquid crystalline materials. During our study of new ionic liquid crystals,5–8 we synthesized novel types of ionic liquid crystalline amphiphiles in which a hydrophilic poly(ethyleneimine) unit with hydroxy side-groups is attached to an aromatic mesogenic core. They showed amphotropic behavior. Here we describe the phase transitions and orientational behavior in the thermotropic and lyotropic liquid crystalline systems containing these novel amphiphilic compounds.
The ionic amphiphilic compounds prepared by us were obtained by ring-opening polymerization of aziridine-1-ethanol (AE) using 6-bromo-1-[4-(4-nitrophenylazo)phenoxy]hexane (Br6N) [or 12-bromo-1-[4-(4-nitrophenylazo)phenoxy)dode- cane (Br12N)] as the polymerization initiator. AE (0.05 or 0.07 mol) and Br6N (0.01 mol) were dissolved in CHCl3, and the mixture was stirred for 300 h at room temperature. After the reaction, the solution was concentrated and diluted in THF. The target material precipitated from the solution and was filtered off. The degree of polymerization (n) of the poly(ethyleneimine) unit was estimated by 1H NMR. The ratio of the numbers of protons involved in methylene groups directly attached to the hydroxy group (3.7 ppm) and the phenyl ring (7.05 ppm) was examined. The existence of a terminal ionic group was shown by the peak at 3.5 ppm in the 1H NMR spectra. The phase transitions of the samples were examined by DSC (Mettler DSC-20 and Shimadzu DSC-50Q) and polarizing microscopy (Nikon polarizing microscope equipped with a Mettler hot stage FP82). The X-ray diffraction measurements were performed using a Rigaku Rint 2100 X-ray diffractometer with Ni-filtered Cu-Kα radiation at various temperatures; the samples were inserted into a Linkam hot stage HFS91.
The phase transition temperatures of the thermotropic liquid crystalline systems are summarized in Table 1. The amphiphilic liquid crystals (6E-6N, 4E-6N) having a hexamethylene linkage chain showed a thermotropic smectic A phase upon heating and cooling. Both 6E-6N and 4E-6N formed focal conics and a perpendicular alignment in the smectic A phase. The amphiphilic liquid crystal (3E-12N) with a dodecamethylene spacer unit also exhibited a thermotropic smectic A phase with focal conic fan and oily streak textures. The smectic A liquid crystal formation in 6E-6N, 4E-6N and 3E-12N is due to the aggregation of the hydrophilic poly(ethyleneimine) units and an overlapping arrangement of the aromatic mesogenic groups due to the strong interaction of the nitro terminal groups.
In this study, the lyotropic systems of 3E-12N were prepared, and their lyotropic liquid crystalline properties were estimated. 3E-12N was water-miscible and easily dissolved in water. The lyotropic systems (L1) consisting of 3E-12N and water formed a lyotropic smectic C phase, as well as the lyotropic smectic A phase (Fig. 1). In L1, focal conics and a perpendicular alignment were formed upon cooling from the isotropic phase to the lyotropic smectic A phase, and in the lyotropic smectic C phase, broken fan and schlielen textures clearly appeared (Fig. 2).
 | ||
| Fig. 1 Phase diagram of lyotropic system (L1) of 3E-12N. | ||
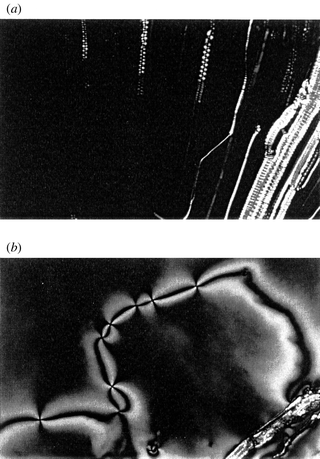 | ||
| Fig. 2 Optical textures of L1 (50wt%): (a) lyotropic smectic A phase (70 °C); (b) lyotropic smectic C phase (50 °C). | ||
The lyotropic smectic C phase is induced by the addition of water to the amphiphilic 3E-12N because anhydrous 3E-12N cannot form the smectic C phase by itself. It is common that lyotropic liquid crystals form nematic, smectic A-like lamellar, columnar and cubic mesophases. However, a tilted smectic type of lyotropic mesophase, such as a smectic C phase, is seldom formed, and only a few reports have described the observation of the lyotropic smectic C phase for nonionic amphiphile/water mixtures.9 The formation of the lyotropic smectic C phase in L1 is a rare case and is probably related to the balance of the hydrophilic and hydrophobic units in the 3E-12N molecule.
The X-ray diffraction pattern of thermotropic 3E-12N consists of sharp reflections at small angles and a broad reflection in the wide-angle region. The layer spacing of thermotropic 3E-12N was 44 Å. In the thermotropic smectic A structure with hydrophobic and hydrophilic sublayers, the nitroazobenzene groups form the interdigitated structure, and poly(ethyleneimine) groups aggregate through hydrogen bondings and ionic interactions.
In the lyotropic mesophase, L1 showed X-ray diffraction patterns consisting of sharp reflections at small angles and a broad reflection in the wide-angle region (Fig. 3).
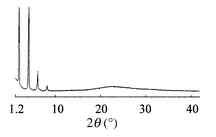 | ||
| Fig. 3 X-Ray diffraction pattern for lyotropic smectic C phase of L1 (50 wt%) at 45 °C. | ||
In L1 (50 wt%), the smectic A and C layer spacings were 50 (65 °C) and 45 Å (45 °C), respectively. In the lyotropic smectic phases, the nitroazobenzene mesogenic units overlap each other, and the poly(ethyleneimine) units and water aggregate through hydrogen bonding. It is expected that the layer structure of the lyotropic smectic A phase resembles that of the thermotropic smectic A phase. In the lyotropic smectic C phase, the hydrophobic sublayers, in which the mesogenic units were tilted with respect to the layer normal, are separated by the hydrophilic units and water.
References
- Handbook of Liquid Crystals, ed. D. Demus, J. Goodby, G. W. Gray, H.-W. Spiess and V. Vill, Wiley-VCH, Weinheim, 1998, vol. 3, ch. VI and VII. Search PubMed.
- H. Ringsdorf, B. Schlarb and J. Venzmer, Angew. Chem., Int. Ed. Engl., 1988, 27, 115 CrossRef.
- D. W. Bruce, D. A. Dunmur, E. Lalinde, P. M. Maitlis and P. Styring, Nature, 1986, 323, 791 CrossRef CAS; D. W. Bruce and S. A. Hudson, J. Mater. Chem., 1994, 4, 479 RSC.
- E. Bravo-Grimaldo, D. Navarro-Rodriguez, A. Skoulios and D. Guillon, Liq. Cryst., 1996, 20, 393 CAS.
- S. Ujiie, S. Takagi and M. Sato, High Perform. Polym., 1998, 10, 347 CrossRef.
- Y. Haramoto, S. Ujiie and M. Nanasawa, Liq. Cryst., 1996, 21, 923 CAS.
- S. Ujiie and K. Iimura, Macromolecules, 1992, 25, 3174 CrossRef CAS.
- S. Ujiie and K. Iimura, Polym. J., 1993, 25, 347 Search PubMed.
- M. A. Schafheutle and H. Finkelmann, Liq. Cryst., 1988, 3, 1369 CAS; N. Pictschmann, A. Lunow, G. Brezesinski, C. Tschierske, F. Kuschel and H. Zaschke, Colloid Polym. Sci., 1991, 269, 636.
| This journal is © The Royal Society of Chemistry 2000 |

