Novel liquid-crystalline PPE-naphthalene copolymers displaying blue solid-state fluorescence
Neil G. Pschirer, Mary E. Vaughn, Y. B. Dong, Hans-Conrad zur Loye and Uwe H. F. Bunz*
Department of Chemistry and, Biochemistry, The University of South Carolina, Columbia, South Carolina 29208, USA.. E-mail: bunz@psc.sc.edu
First published on UnassignedUnassigned6th January 2000
Abstract
Synthesis of poly(p-phenyleneethynylene)s (PPEs) containing 1,5-diethynyl-3,7-di-tert-butylnaphthalene leads to novel phenylene-naphthylene-ethynylene copolymers which show strong blue luminescence in the solid state.
Conjugated polymers are organic semiconductors and find inter alia applications in electroluminescent devices1,2 and ‘plastic lasers’.3 We have recently reported an efficient synthesis of high molecular weight poly(p-phenyleneethynylene)s (PPEs) by alkyne metathesis utilizing simple ‘instant catalysts’.4 PPEs are promising materials, because they show quantum yields of 1.0 and blue emission in dilute solution. However, in the solid state, the emission spectra of PPEs are much weaker and considerably red-shifted as a consequence of aggregation and concomitant excimer formation in the solid state.5,6 Likewise poly(3,7-di-tert-butylnaphthyleneethynylene), which we recently synthesized, is effectively non-fluorescent as film or powder.7
Herein we repot the synthesis and characterization of the unsaturated copolymers 3 which are efficient blue emitters in the solid state (Fig. 1). The polymers 3 are obtained by adding defined amounts of 3,7-di-tert-butyl-1,5-dipropynylnaphthalene 27 to 1,4-dipropynyl-2,5-dialkylbenzenes 1 (Scheme 1) in our alkyne metathesis protocol utilizing an instant catalyst formed of Mo(CO)6 and 4-chlorophenol.8† The catalyst and a varying ratio of monomers 1 and 2 were stirred in 1,2-dichlorobenzene at 140 °C for 11–25 h under a flow of N2. This protocol results in a series of high molecular weight copolymers 3. Longer polymerization times give rise to higher degrees of polymerization, as examined in the PPE system in detail.8
 | ||
| Fig. 1 Solid-state emission (hand held fluorescence lamp, λmax = 366 nm) of PPE and PPE-napthalene copolymers 3: (a) didodecyl-PPE; (b) polymer 3a; (c) polymer 3c; (d) polymer 3d; (e) poly(3,7-di-tert-butylnaphtyleneethynylene). | ||
 | ||
| Scheme 1 | ||
The UV–VIS spectra of 3a–d in dilute solution show a λmax of 388–395 nm, very similar to that of dialkyl-PPEs (388 nm). To our surprise, thin film absorption spectra of 3a–d are identical to those in solution, which is in stark contrast to the PPE case where a dramatic red-shift is observed. The unusual optical behavior prompted us to investigate fluorescence of 3a–d. In solution an intense blue emission is observed (3a, 425 nm; 3d, 419 nm), again similar to dialkyl-PPEs (425 nm). In the solid state, however, the situation is different, and in thin films of 3a–d we find emission maxima ranging from 446 (5∶1, 3a) to 422 nm (1∶2, 3d), (Fig. 2), thus allowing the manipulation of solid-state emission maximum from yellow–green to blue via the amount of added monomer 2. The less PPE character the copolymer 3 has, the further its emission is blue-shifted towards that observed in dilute solution. We conclude that in the copolymers 3 aggregation and supramolecular ordering are efficiently suppressed by the presence of the bulky tert-butylnaphthyl groups.9 This scenario must lead to a disordered solid state in 3. To test this hypothesis we performed powder diffraction on polymers 3 (Fig. 3). The polymers 3a–c display a broad diffraction peak of large intensity according to d = 4.2 Å. This diffraction peak can be attributed to the interchain distance of the polymers. A second, weak diffraction peak is observed at d = 8.8–11.7 Å, but is very weak in 3a–c. In the naphthalene-rich polymer 3d the small-angle diffraction at 11.7 Å is most prevalent. Molecular modeling indicates that this represents the distance between the two 3,7-tert-butyl groups on individual naphthalene units.10
 | ||
| Fig. 2 Solid-state emission spectra of thin films of 3a–d and PPE. | ||
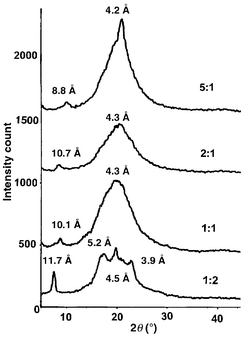 | ||
| Fig. 3 Diffraction data of polymers 3a–d. | ||
For device purposes, liquid crystalline behavior offers the entry to polarized emission. DSC and polarizing microscopy performed upon 3a–c shows that 3a,b are thermotropic nematic (3a: isotropic → nematic 160 °C DSC; nematic → isotropic 170 °C → nematic 149 °C, evidenced by polarizing microscopy, cooling. 3b: 194 °C polarizing microscopy, cooling isotropic → nematic), both displaying Schlieren textures. Copolymer 3c displays a broad halo at 4.3 Å, and a weak small angle feature, suggesting either small domain sizes or a disordered structure. No identifiable textures develop upon thermal treatment. Only in the case of 3a is the isotropic → nematic transition observed in the DSC and it is exothermic, with 0.27 kcal mol−1 per repeating unit, a small but not unexpected value.11 Polymer 3d is crystalline and does not show any phase transition up to 300 °C.
In conclusion we have presented the synthesis and characterization of a new series of liquid-crystalline PPE copolymers 3 which exhibit blue solid state fluorescence. The blue shift increases with the amount of naphthalene. Particularly attractive is the intensity of luminescence of 3 in the solid state. The increased solid-state fluorescence is attributed to the absence of aggregates and excimers, which normally reduce PPE’s quantum efficiencies of emission in the solid state. Application of 3 in LEDs and sensory materials12 is currently being explored.
Acknowledgements
We acknowledge Professor C. J. Murphy and L. Gearhart for help with the fluorescence spectra. This work was generously supported by the NSF (CHE 9814118, PI Bunz) and the Research Corporation.References
- D. Neher, Adv. Mater., 1995, 7, 691 CAS; A. Kraft, A. C. Grimsdale and A. B. Holmes, Angew. Chem., 1998, 37, 402 CrossRef; J. H. Burroughes, D. D. C. Bradley, A. R. Brown, R. N. Marks, K. Mackay, R. H. Friend, P. L. Burns and A. B. Holmes, Nature, 1990, 347, 539 CrossRef CAS.
- S. A. Jenekhe and J. A. Osaheni, Science, 1994, 265, 765 CrossRef CAS; J. A. Osaheni and S. A. Jenekhe, Macromolecules, 1994, 27, 739 CrossRef; X. Zhang, A. S. Shetty and S. A. Jenekhe, Acta Polym., 1998, 49, 52 CrossRef CAS; E. Conwell, Trends Polym. Sci., 1997, 5, 218 Search PubMed.
- F. Hide, M. A. Diaz-Garcia, B. J. Schwartz and A. J. Heeger, Acc. Chem. Res., 1997, 30, 430 CrossRef CAS.
- L. Kloppenburg, D. Song and U. H. F. Bunz, J. Am. Chem. Soc., 1998, 120, 7973 CrossRef CAS.
- C. E. Halkyard, M. E. Rampey, L. Kloppenburg, S. L. Studer-Martinez and U. H. F. Bunz, Macromolecules, 1998, 31, 8655 CrossRef CAS; R. Fiesel, C. E. Halkyard, M. E. Rampey, L. Kloppenburg, S. L. Studer-Martinez, U. Scherf and U. H. F. Bunz, Macromol. Rapid Commun., 1999, 20, 107 CrossRef CAS.
- D. Ofer, T. M. Swager and M. S. Wrighton, Chem. Mater., 1995, 7, 418 CrossRef CAS; Q. Zhou and T. M. Swager, J. Am. Chem. Soc., 1995, 117, 12593 CrossRef CAS; C. Weder and M. S. Wrighton, Macromolecules, 1996, 29, 5157 CrossRef CAS.
- N. G. Pschirer, A. Marshall, C. Stanley, H. W. Beckham and U. H. F. Bunz, Macromol. Rapid Commun., 2000, 21, in the press. Search PubMed.
- L. Kloppenburg, D. Jones and U. H. F. Bunz, Macromolecules, 1999, 32, 4194 CrossRef CAS.
- B. R. Hsieh, W. C. Wan, Y. Yu, Y. L. Gao, T. E. Goodwin, S. A. Gonzalez and W. A. Feld, Macromolecules, 1998, 31, 631 CrossRef CAS; W. J. Oldham, R. J. Lachicotte and G. C. Bazan, J. Am. Chem. Soc., 1998, 120, 2987 CrossRef CAS; G. C. Bazan, Y. J. Miao, M. L. Renak and B. J. Sun, J. Am. Chem. Soc., 1996, 118, 2618 CrossRef CAS; Z. H. Peng, J. H. Zhang and B. B. Xu, Macromolecules, 1999, 32, 5152.
- U. H. F. Bunz, V. Enkelmann, L. Kloppenburg, D. Jones, K. D. Shimizu, J. B. Claridge, H.-C. zur Loye and G. Lieser, Chem. Mater., 1999, 11, 1416 CrossRef CAS; L. Kloppenburg, D. Jones, J. B. Claridge, H. C. zur Loye and U. H. F. Bunz, Macromolecules, 1999, 32, 4460 CrossRef CAS.
- M. Altmann and U. H. F. Bunz, Angew. Chem., 1995, 35, 569.
- J. S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 11864 CrossRef CAS.
Footnote |
| † Sample coploymerization: 1 (0.35 g, 0.71 mmol), 2 (0.24 g, 0.71 mmol), Mo(CO)6 (0.034 g, 0.07 mmol), 4-chlorophenol (0.091 g, 0.71 mmol) and 1,2-dichlorobenzene (20 mL) were held at 140 °C under a steady stream of N2 for 17 h. Addition of chloroform acid and base washes and precipitation into methanol furnish 3c in 80% yield. Selected data for 3c: δH 8.58–8.54 (m), 8.07 (br s), 7.94 (br s), 7.60–7.42 (m), 3.02–2.89 (m), 2.13 (br s), 2.12 (br s), 1.79–1.68 (m), 1.51–1.22 (m), 0.88–0.87 (br s); δC 148.4, 141.9, 132.5, 131.3, 123.0, 122.8, 121.1, 93.5, 92.7, 35.1, 34.4, 32.0, 31.4, 31.3, 30.8, 29.8, 29.5, 29.4, 22.8, 14.2; νmax(KBr)/cm−1 3444, 2957, 2923, 2853, 2350, 1651, 1463, 1384, 1025, 886, 771. |
| This journal is © The Royal Society of Chemistry 2000 |
