Cationic palladium(II) complex-catalyzed [2 + 2] cycloaddition and tandem cycloaddition–allylic rearrangement of ketene with aldehydes: an improved synthesis of sorbic acid
Tetsutaro Hattori*, Yutaka Suzuki, Osamu Uesugi, Shuichi Oi and Sotaro Miyano*
Department of Biomolecular Engineering, Graduate School of Engineering, Tohoku University, Aramaki-Aoba 07, Aoba-ku, Sendai, 980-8579, Japan.. E-mail: hattori@orgsynth.che.tohoku.ac.jp
First published on UnassignedUnassigned7th January 2000
Abstract
Cationic palladium(II) complexes [PdL2(PhCN)2](BF4)2 efficiently catalyze the [2 + 2] cycloaddition of ketene with aldehydes to give the corresponding oxetan-2-ones, among which 4-vinyl-substituted ones are further isomerized under the conditions to give 3,6-dihydro-2H-pyran-2-ones in good yields.
There has been much current interest in the preparation of oxetan-2-ones (β-lactones) because they are not only structural units in biologically active natural products but also versatile synthetic intermediates.1 Among various synthetic routes to this class of compounds, the most efficient and concise is the Lewis acid-catalyzed [2 + 2] cycloaddition of ketenes with aldehydes.1 Recently, we have reported the first asymmetric version of the cycloaddition mediated by a catalytic amount of Corey’s aluminium-based bissulfonamides.2 However, the reaction requires the use of at least 10 mol% of the catalyst to obtain the lactones in practical yields, as has been revealed in the related reactions catalyzed by typical metal-based Lewis acids. This may be ascribed to the highly oxophilic nature of the acid, which causes competitive ligation between the reactants and the product to the catalyst to reduce the catalytic efficiency. Deactivation of the catalyst by a trace amount of incidental water in the reaction system is another plausible problem.
On the other hand, it has been recognized in the last decade that certain transition metal complexes have considerable Lewis acid character and can displace conventional Lewis acids in a variety of the so-called Lewis acid-catalyzed reactions.3 Furthermore, some of the transition metal-based Lewis acids are reported to be effective even in the presence of water.4 These facts prompted us to examine whether this class of Lewis acids can effect the cycloaddition reaction. Herein, we report a highly efficient [2 + 2] cycloaddition of ketene with aldehydes 1 using cationic palladium(II) complexes [PdL2(PhCN)2](BF4)225 as the catalyst (Scheme 1).
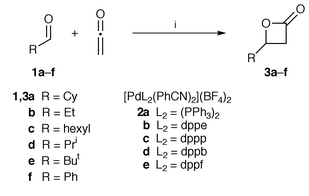 | ||
| Scheme 1 Reagents and conditions: i, 2, CH2Cl2. dppp = 1,3-bis(diphenylphosphino)propane, dppf = 1,1′-bis(diphenylphosphino)ferrocene. | ||
The general procedure for the [2 + 2] cycloaddition is as follows (Method A): to a solution of complex 2 (50.0 μmol) in dry CH2Cl2 (20 cm3) was added aldehyde 1 (1.00 mmol) under nitrogen at an appropriate temperature. Gaseous ketene (ca. 2.5 mmol) was bubbled into the mixture over a period of 5 min and the resulting mixture was stirred at this temperature for 1 h. After usual work-up, the crude product was subjected to GC analysis to determine the yield of lactone 3.
The results are listed in Table 1. The reaction of cyclohexanecarbaldehyde 1a with ketene in the presence of 5 mol% of a palladium complex 2a–e proceeded even at −78 °C to give the lactone 3a (entries 1–5). The catalytic activity of the palladium complexes 2a–e varied depending on the coordinating phosphine ligands, among which dppb (2d) was the most effective. Addition of powdered molecular sieves (3 Å) did not improve the yield of lactone 3a (entry 6). An irregular temperature dependence of the product yield was found in the reaction conducted at −40 °C (entry 7, as compared with entries 4, 8 and 9). This may be ascribed to the balance between an increase in the rate constant with the rise in the reaction temperature and a significant decrease in the concentration of ketene around its boiling point (−41 °C). Lactone 3a was obtained in quantitative yield at room temperature, even if the quantity of the catalyst was reduced to 1 mol% (entry 10). Judging from the high catalytic activity and the lack of apparent effect of the dehydrating agent (entry 6), the catalyst seems to be compatible with the trace amounts of water in the system. The reaction of several other aldehydes 1b–f with ketene afforded the corresponding lactones 3b–f in good to excellent yields (entries 11–15).
| Entry | 1 | 2 | T/°C | 3 | Yield (%)a |
|---|---|---|---|---|---|
| a Determined by GC analysis on ASTEC Chiraldex G-TA column (0.25 mm i.d. × 20 m) by the internal standard method.b Powdered molecular sieves (3 Å) (100 mg) were added.c 1.0 mol%.d Isolated yield of the 1,3-diol derived from compound 3f. | |||||
| 1 | 1a | 2a | −78 | 3a | 33 |
| 2 | 1a | 2b | −78 | 3a | 46 |
| 3 | 1a | 2c | −78 | 3a | 55 |
| 4 | 1a | 2d | −78 | 3a | 66 |
| 5 | 1a | 2e | −78 | 3a | 56 |
| 6 | 1a | 2d | −78 | 3a | 63b |
| 7 | 1a | 2d | −40 | 3a | 52 |
| 8 | 1a | 2d | 0 | 3a | 97 |
| 9 | 1a | 2d | room temp. | 3a | 98 |
| 10 | 1a | 2dc | room temp. | 3a | 99 |
| 11 | 1b | 2d | room temp. | 3b | 99 |
| 12 | 1c | 2d | room temp. | 3c | 97 |
| 13 | 1d | 2d | room temp. | 3d | 99 |
| 14 | 1e | 2d | room temp. | 3e | 63 |
| 15 | 1f | 2d | room temp. | 3f | 61d |
Next, our interest was directed toward the possibility of using the palladium complex 2d as the catalyst for the cycloaddition of ketene with α,β-unsaturated aldehydes 1g–k (Scheme 2). The reaction of ketene with crotonaldehyde 1g under the standard conditions (Method A, vide supra) afforded not the β-lactone 3g but a δ-lactone, 3,6-dihydro-6-methyl-2H-pyran-2-one (isoparasorbic acid) 4g,6 though only in poor yield, along with an unidentifiable polymer (vide infra) (Table 2, entry 1).7 Lowering the reaction temperature and changing the molar ratios of ketene and catalyst 2d to aldehyde 1g did not improve the product yield, while dilution of the reaction solution was found to be highly effective (entry 2). Eventually, lactone 4g could be obtained in good yields by adding aldehyde 1g and ketene portionwise to a dilute solution of catalyst 2d (Method B† ) (entries 3 and 5). Under these conditions, 2.5 mol% of the catalyst 2d was sufficient to complete the reaction (compare entry 6 with entry 3). Similar δ-lactones 4i–k were also obtained in the reaction of α,β-unsaturated aldehydes 1i–k, while acrolein 1h afforded β-lactone 3h under the same conditions (entries 7–10).
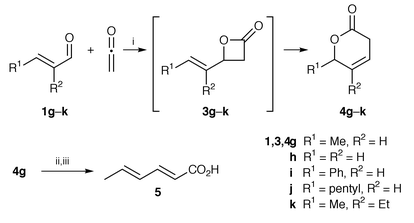 | ||
| Scheme 2 Reagents and conditions: i, 2d, CH2Cl2; ii, EtOH, conc. HCl, reflux; iii, KOH, aqueous EtOH, reflux. | ||
| Entry | 1 | Methoda | CH2Cl2/cm3 | 4 | Yield (%)b |
|---|---|---|---|---|---|
| a See text.b Determined by GC analysis on Quadrex MPS-10 column (0.32 mm i.d. × 25 m) by the internal standard method.c Isolated yield after column chromatography on silica gel with hexane–EtOAc (1∶1) as the eluent.d 2.00 mmol.e Lactone 3h was obtained in 96% yield. | |||||
| 1 | 1g | A | 20 | 4g | 13 |
| 2 | 1g | A | 200 | 4g | 55 |
| 3 | 1g | B | 200 | 4g | 70 |
| 4 | 1g | B | 200 | 4g | 50c |
| 5 | 1g | B | 500 | 4g | 81 |
| 6 | 1gd | B | 200 | 4g | 65 |
| 7 | 1h | B | 200 | 4h | 0e |
| 8 | 1i | B | 200 | 4i | 77c |
| 9 | 1j | B | 200 | 4j | 58c |
| 10 | 1k | B | 200 | 4k | 66c |
The formation of lactone 4 can be rationalized by the initial [2 + 2] cycloaddition of aldehyde 1 with ketene to give the allyl ester 3, followed by its allylic rearrangement to form lactone 4. It is known that this type of 1,3-rearrangement of allylic esters is promoted by Pd0 and PdII complexes.8 It should be noted, however, that the palladium(II)-catalyzed reaction is reportedly a [3,3]-sigmatropic rearrangement of allyl esters, which is impossible for the said lactones 3g–k due to steric reasons. On the other hand, the palladium(0)-catalyzed rearrangement is believed to involve a π-allylpalladium(II) intermediate. It is also reported that palladium(II) salts promote the ring opening of 4-vinyl- (3h), and 4-isopropenyl-oxetan-2-one to afford the corresponding penta-2,4-dienoic acids.9 A metallacyclic σ-allylpalladium intermediate generated by oxidative addition of the C(4)–O bond of the oxetan-2-ones to a palladium(0) species is proposed for the reaction. Thus, an allylpalladium species may be a possible intermediate for the present reaction. We found, however, that BF3·OEt2 also catalyzed the rearrangement to give lactone 4g, though only in 13% yield, when aldehyde 1g was treated with ketene (method B) in Et2O (200 cm3) at room temperature in the presence of 1.5 equiv. of the acid.10 This observation, along with the result that lactone 3h did not isomerize to lactone 4h, may suggest another possibility, that coordination of the carbonyl oxygen of 4-vinyl lactones 3g, i–k to Lewis acid 2d promoted the heterolytic cleavage of the C(4)–O bond of the lactones to form a zwitterion, recombination of which at the other allylic terminus afforded lactones 4.
Further treatment of lactone 4g with EtOH in the presence of HCl followed by saponification of the resulting ethyl sorbate, gave hexa-2,4-dienoic acid (sorbic acid) 5 in 90% yield (Scheme 2). Therefore, the present method provides an easy access to the acid 5. It should be noted that sorbic acid 5 is important as a mould and yeast inhibitor, the first step of an industrial synthesis of which relies on the cycloaddition of ketene with crotonaldehyde 1g catalyzed by a zinc carboxylate.11 However, the product obtained from the reaction is not the β-lactone 3g but its ring-opening polymer, poly(3-hydroxyhex-4-enoic acid), the viscosity of which causes a great deal of trouble during the subsequent destructive distillation of the polyester to the acid 5. Further studies on the scope and limitations of the allylic rearrangement, as well as the [2 + 2] cycloaddition, are in progress.
Acknowledgements
This work was supported in part by grants from the Center of Interdisciplinary Research (Tohoku University), the Takasago International Corporation and the Chisso Corporation.References
- Review: A. Pommier and J.-M. Pons, Synthesis, 1993, 441. Search PubMed.
- Y. Tamai, H. Yoshiwara, M. Someya, J. Fukumoto and S. Miyano, J. Chem. Soc., Chem. Commun., 1994, 2281 RSC.
- Review: B. Bosnich, Aldrichim. Acta, 1998, 31, 76. Search PubMed.
- W. Odenkirk, A. L. Rheingold and B. Bosnich, J. Am. Chem. Soc., 1992, 114, 6392 CrossRef CAS; S. Kanemasa, Y. Oderaotoshi, S. Sakaguchi, H. Yamamoto, J. Tanaka, E. Wada and D. P. Curran, J. Am. Chem. Soc., 1998, 120, 3074 CrossRef CAS.
- J. A. Davies, F. R. Hartley and S. G. Murray, J. Chem. Soc., Dalton Trans., 1980, 2246 RSC; S. Oi, K. Kashiwagi, E. Terada, K. Ohuchi and Y. Inoue, Tetrahedron Lett., 1996, 37, 6351 CrossRef CAS.
- R. Stevenson and J. V. Weber, J. Nat. Prod., 1988, 51, 1215 CrossRef CAS.
- Only one report was found in literature, which described a formation of similar δ-lactones in the cycloaddition of ketene with ketones: F. G. Young, J. Am. Chem. Soc., 1949, 71, 1346. Search PubMed.
- Reviews: R. P. Lutz, Chem. Rev., 1984, 84, 205; Search PubMed; L. E. Overman, Angew. Chem., Int. Ed. Engl., 1984, 23, 579 Search PubMed.
- A. F. Noels, J. J. Herman and P. Teyssié, J. Org. Chem., 1976, 41, 2527 CrossRef CAS.
- Hagemeyer reported that the boron trifluoride-catalyzed reaction of ketene with crotonaldehyde 1g gave lactone 3g: H. J. Hagemeyer, Jr., US 2,478,388/1949 (Chem. Abstr., 1950, 44, 1132). See also: J. H. McCain and E. Marcus, J. Org. Chem., 1970, 35, 2414..
- H. J. Hagemeyer Jr., Ind. Eng. Chem., 1949, 41, 765 Search PubMed.
Footnote |
| † Method B: To a solution of complex 2d (50.0 μmol) in CH2Cl2 (200 or 500 cm3) was added aldehyde 1 (200 μmol). Ketene (ca. 250 μmol) was bubbled into the mixture over a period of 1 min and the mixture was stirred for 5 min. This series of operations was repeated until added aldehyde 1 reached the total amount of 1.00 mmol. To the mixture was added an additional amount of ketene (ca. 1.0 mmol) and the resulting mixture was stirred for 1 h before work-up. |
| This journal is © The Royal Society of Chemistry 2000 |
