Study of the accessibility of phosphorus centres incorporated within ordered mesoporous organic–inorganic hybrid materials
Robert J. P.
Corriu
*,
Christian
Hoarau
,
Ahmad
Mehdi
and
Catherine
Reyé
Laboratoire de Chimie Moleculaire et Organisation du
Solide, UMR 5637 CNRS, Université de Montpellier II, Sciences et Techniques du
Languedoc, Place E. Bataillon, F-34095, Montpellier Cedex 5, France.. E-mail: reye@crit.univ-montp2.fr
First published on 6th January 2000
Abstract
Phosphorus centres incorporated within surfactant-directed mesoporous hybrid materials are shown to be more easily accessible than those incorporated within the corresponding materials prepared in the absence of surfactant.
The one step preparation of organically functionalised ordered mesoporous silica by using non-ionic1,3 and ionic surfactants4–9 as structure-directing agents constitutes a new and very promising route to hybrid materials with organised functionalities. Our contribution in this field was the preparation of surfactant-directed mesoporous hybrid materials incorporating phosphino groups.3 We showed that such materials are easily accessible and undergo further chemical reactions at phosphorus (sulfuration and quaternization) without disrupting the ordered structure and without changing notably the textural characteristics, thus suggesting the location of the P centres within the mesopore channels.5 The potential applications of these materials containing P centres for catalysis10 and separation which are strongly connected with the chemical accessibility of the functionalities have led us to further studies. Here, we show that, in surfactant-directed mesoporous hybrid materials, phosphorus centres are more easily accessible than in the corresponding materials prepared in the absence of surfactants.
The hybrid materials Xn and X′n were obtained by co-hydrolysis and polycondensation of mixtures Ph2P(CH2)3Si(OMe)3/nS i(OEt)4 (n = 6, 9 and 19) in the presence of n-hexadecylamine as template for Xn and in the absence of template but in the presence of 1% TBAF as catalyst for X′n (Scheme 1). The molar composition of each mixture was: 1 − x Si(OEt)4:xPh2P(CH2)3S i(OMe)3: 0.27 n-hexadecylamine∶24.2 H2O∶9.1 EtOH. The xerogels were prepared according to previously published procedures3 and some relevant physical properties are given in Table 1. The BET surface areas were determined by N2 adsorption–desorption isotherm measurements. Xn (n = 6, 9, 19) exhibit type IV isotherms,11 characteristic for mesoporous materials. In contrast, the isotherms of X′n are indicative of macroporosity.11 XRD patterns for Xn exhibit a single diffraction peak corresponding to d100 spacing while no peak was observed for X′n. Organic incorporation was calculated by thermogravimetric analysis. It is worth noting that the calculated values are slightly closer to theoretical values for the mesoporous xerogels than for the others and this, in particular for high ‘dilutions’ of the organic moiety in silica. Another interesting observation in relation to the effect of dilution was made from the 31P NMR data of the materials. As shown in Fig. 1, the 31P NMR spectra for X′n displayed signals which are always broader than those for Xn. This is an indication of a greater mobility for the P centres in Xn than in X′n. Furthermore, on going from the ‘dilution’ n = 9 to the higher ‘dilution’ n = 19, the Dn1/2 values remain stable for the ordered hybrid materials while for the others X′n, they regularly decrease as the ‘dilution’ increases. Thus it can be concluded that from n = 9 all the P centres within Xn have the same surroundings, the organic pendants being regularly dispersed at the surface of the mesopores while for the solids X′n the distribution of the organic groups is random.
 | ||
| Scheme 1 | ||
| Xerogel | d 100 Lattice spacing/Å | BET surface area/m2 g−1 | Total pore volume/cm3 g−1 | Pore diametera/Å | 31P CP MAS NMR (δ) | Organic incorporationb (%) | |
|---|---|---|---|---|---|---|---|
| a Measured using the Horvath–Kawazoe pore size distribution model. b Percentage of Si atoms present as organosilane with respect to total Si content calculated by thermogravimetric analysis. c Theoretical values. | |||||||
| X 6 (X′6) | 41.86 (—) | 385 (149) | 0.30 (—) | 27 (≈300) | −15.84 | 14.2c | 11 (10) |
| X 9 (X′9) | 34.41 (—) | 1100 (350) | 0.53 (—) | 35 (≈300) | −15.71 (−15.61) | 10c | 9.4 (8.8) |
| X 19 (X′19) | 38.91 (—) | 1380 (640) | 0.61 (—) | 34 (≈300) | −15.13 (−15.0) | 5c | 5 (4.7) |
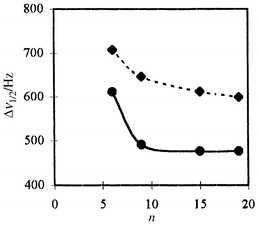 | ||
| Fig. 1 Half linewidth (Δν1/2) of the solid state 31P NMR signals for Xn (———) and X′n (-----) as a function of the ‘dilution’ n. | ||
The accessibility of the P centres in these materials was investigated by using the reactions of quaternization and complexation of phosphorus centres. All the reactions at phosphorus were monitored by solid state 31P NMR spectroscopy which is a reliable and sensitive probe for immobilized phosphorus groups.12
The solids Xn (n = 6, 9, 19) were treated with 1 equiv. of benzyl bromide 1 or 0.5 equiv. of α,α′-dibromoparaxylene 2 per P centre in CH2Cl2 at 20 °C. It is to be noted that after treatment, the 31P NMR spectra of materials exhibited no signal corresponding to the oxidation of the P centres. The results given in Table 2 show that the percentage of quaternization of P centres increase with the dilution of the organic moieties in silica with both reagents. The reaction is quantitative with 1 after 72 h from X9 and X19. It is also quantitative with the bifunctional compound 2 from X19 after 72 h and almost quantitative from X9 which is noteworthy as in that case all the P centres are bridged. The same reactions were carried out on X′n and the percentage of quaternization was calculated after 24 h of reaction. These results are compared to those obtained from Xn after the same reaction time in Table 3. It appears that the percentage of reaction with 1 is always lower from X′n than from Xn, whatever the dilution. Thus the P centres are more easily accessible within ordered mesoporous materials Xn than within the amorphous X′n. Interestingly, with the bifunctional reagent 2, the gap between the percentage of quaternization of P centres obtained from Xn and X′n increases with the dilution in favour of the ordered materials Xn. This result is due to the regular distribution of organic moieties in Xn. Indeed, while the substitution of the second bromide of 2 is promoted by the dilution within Xn, it is rendered difficult within X′n because of the random distribution of the organic groups in silica. The distribution of the organic moieties within the solids was further studied by treating the materials with 0.5 equiv. cis-(PPh3)2PdCl2 per P centre. We observed that the solid state 31P NMR spectra of the materials after 24 h of reaction at 20 °C in CH2Cl2 displayed a resonance at δ −16 corresponding to the remaining starting phosphine and a resonance at δ +16 which was assigned to the trans anchored phosphine PdII complex.12 A further resonance at δ +36 was attributed to starting phosphine oxidation, which never exceeded 15%. Treatment of the materials with a large excess of the nucleophile PBun3 allowed the complete elimination of the palladium liberating the starting phosphine (δ −16). The presence of the unchanged signal at δ +36 after this treatment confirmed the oxidation of the starting phosphine. The extent of the anchored phosphine PdII complex obtained from Xn and X′n determined by 31P NMR spectroscopy is shown in Fig. 2. The difference between the reactivity of materials Xn and X′n (n = 9, 19) is important. Thus the diffusion of a bulky molecule like (PPh3)2PdCl2 through materials X′n is much more difficult than through the ordered mesoporous materials Xn. The very low extent of reaction for X6 compared to X9 and X19 should be due to steric hindrance, which implies also that P centres are mostly located within the mesopores of Xn.
| Reaction time/h | X 6 | X 9 | X 19 |
|---|---|---|---|
| 2 | 48 (35) | 56 (58) | 78 (74) |
| 16 | 63 (68) | 72 (82) | 95 (90) |
| 72 | 83 (83) | 100 (93) | 100 (100) |
| n | 6 | 9 | 19 |
|---|---|---|---|
| X n | 71 (73) | 87 (92) | 94 (95) |
| X′ n | 60 (72) | 64 (82) | 79 (70) |
 | ||
| Fig. 2 Percentage of anchored phosphine PdII complex within Xn (———) and X′n (-----). | ||
In conclusion, we have shown that in surfactant-directed mesoporous hybrid materials, phosphorus centres are more easily accessible than in the corresponding materials prepared in the absence of surfactants. Furthermore the accessibility of phosphorus centres within ordered mesoporous hybrid materials depends on the ‘dilution’ of the organic part in silica. The higher the ‘dilution’, the greater the accessibility of the organic part in particular towards bulky reagents for which a minimum ‘dilution’ (n > 6) seems to be required. The study of hybrid materials with very low concentration of organo groups is in progress.
References
- D. J. Macquarrie, Chem. Commun., 1996, 1961 RSC.
- D. J. Macquarrie, D. B. Jackson, J. E. G. Mdoe and J. H. Clark, New J. Chem., 1999, 23, 539 RSC.
- R. J. P. Corriu, A. Mehdi and C. Reyé, C.R. Acad. Sci. Paris, Sér. IIc, 1999, 35 Search PubMed.
- S. L. Burkett, S. D. Sims and S. Mann, Chem. Commun., 1996, 1367 RSC.
- M. H. Lim, C. F. Blanford and A. Stein, J. Am. Chem. Soc., 1997, 119, 4090 CrossRef CAS.
- C. E. Fowler, S. L. Burkett and S. Mann, Chem. Commun., 1997, 1769 RSC.
- M. H. Lim, C. F. Blanford and A. Stein, Chem. Mater., 1998, 10, 467 CrossRef CAS.
- F. Babonneau, L. Leite and S. Fontlupt, J. Mater. Chem., 1999, 9, 175 RSC.
- S. R. Hall, C. E. Fowler, B. Lebeau and S. Mann, Chem. Commun., 1999, 201 RSC.
- B. F. G. Johnson, S. A. Raynor, D. S. Shephard, T. Mashmeyer, J. Meurig Thomas, G. Sankar, S. Bromley, R. Oldroyd, L. Gladden and M. D. Mantle, Chem. Commun., 1999, 1167 RSC.
- S. J. Gregg and K. S. W. Sing, Adsorption, Surface Area and Porosity, Academic Press,New York, 2nd edn., 1982. Search PubMed.
- R. A. Komoroski, A. J. Magistro and P. P. Nicholas, Inorg. Chem., 1986, 25, 3917 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2000 |
