Ring-closing alkyne metathesis with simple catalyst systems: an access to molecular triangles and rhomboids
Neil G. Pschirer, Wei Fu, Richard D. Adams* and Uwe H. F. Bunz*
Department of Chemistry and Biochemistry, The University of South Carolina, Columbia, South Carolina 29208, USA.. E-mail: bunz@psc.sc.edu
First published on UnassignedUnassigned7th January 2000
Abstract
Treatment of the siloxane monomer 2 with a mixture of molybdenum hexacarbonyl and 4-chlorophenol at 140 °C furnished the corresponding cyclotrimer 3 and the cyclotetramer 4.
Ring-closing alkene metathesis utilizing well-defined organometallic catalysts1–4 has developed into a powerful synthetic tool. A variety of rings have been prepared by this method. Cyclic polyynes are also of great interest,5 but their synthesis via ring-closing alkyne metathesis is much less explored.6 Fürstner7 has reported the preparation of large-ring alkynes by ring-closure of suitable dipropynylated precursors utilizing Schrock’s tungsten carbyne.8
We have recently developed an efficient and simple protocol for alkyne metathesis9,10 and we now report the first examples of ring-closing metathesis using our ‘instant’ catalyst. This powerful catalyst is formed in situ from Mo(CO)6 and 4-chlorophenol using off-the-shelf quality solvents.
The synthesis of shape-persistent macrocycles, potentially useful as molecular boxes, presents a new and interesting challenge.11,12 We have chosen diyne 2 to be a convenient precursor to the novel molecular siloxane triangles/boxes 3 and 4. Compound 2 was prepared in two steps from 1, which was, itself, obtained from 4-iodophenol by treatment with Pri2SiCl2 in Et3N (Scheme 1). A palladium-catalyzed coupling of 1 with propyne affords the monomer 2 in 61% yield.13 Alkyne metathesis of 2 with Mo(CO)6 and 4-chlorophenol at 140 °C in 1,2-dichlorobenzene under a steady stream of N2 (Scheme 1) furnished the novel cycles 3 and 4.† Sufficient dilution is an important factor for obtaining cyclic rather than polymeric products, although minor changes in concentration did not result in decreased yields of the macrocycles. Separation was difficult due to the similar retention times of 3 and 4, and their corresponding open congeners. However, we were able to isolate 3 (14% yield) and 4 (18% yield). GC-MS confirmed that the isolated fractions of 3 and 4 were uncontaminated by other cycloisomers. The molecular structures of 3 and 4 were established by single crystal X-ray diffraction analyses.‡
 | ||
| Scheme 1 Reagents and conditions: i, 4-IC6H4OH, Et3N, THF; ii, propyne, Pd(PPh3)2Cl2, CuI, piperidine; iii, Mo(CO)6, 4-ClC6H4OH. | ||
The crystal of 3 contains three symmetry-independent molecules. All three display similar conformations and the molecular structure of one of these is shown in Fig. 1. The cyclotetramer 4 is crystallographically centrosymmetric. It exhibits a rhomboidal structure with a large interior cavity that contains two molecules of hexane as solvate, from which it was crystallized (Fig. 2). This feature indicates the potential of these box-shaped molecules to engage in important host–guest chemistry.
 | ||
Fig. 1 A structural diagram of the cyclic triyne 3. Selected bond
distances (Å) and angles (°) are C![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) C = 1.22(2), 1.20(2)
and 1.16(2), Si(1)–O(1) = 1.616(9), Si(1)–O(2) = 1.624(9),
Si(2)–O(5) = 1.67(1), Si(2)–O(6) = 1.63(1), Si(3)–O(3) =
1.63(1), Si(3)–O(4) = 1.66(1); O(1)–Si(1)–O(2) =
109.8(5), O(5)–Si(2)–O(6) = 106.9(5),
O(3)–Si(3)–O(4) = 108.1(6). C = 1.22(2), 1.20(2)
and 1.16(2), Si(1)–O(1) = 1.616(9), Si(1)–O(2) = 1.624(9),
Si(2)–O(5) = 1.67(1), Si(2)–O(6) = 1.63(1), Si(3)–O(3) =
1.63(1), Si(3)–O(4) = 1.66(1); O(1)–Si(1)–O(2) =
109.8(5), O(5)–Si(2)–O(6) = 106.9(5),
O(3)–Si(3)–O(4) = 108.1(6). | ||
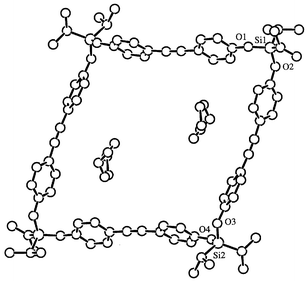 | ||
Fig. 2 A structural diagram of the cyclic tetrayne 4 showing two
molecules of occluded hexane in the interior of the ring. Selected bond
distances (Å) and angles (°) are C![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) C = 1.179(9) and
1.213(9), Si(1)–O(1) = 1.635(4), Si(1)–O(2) = 1.646(4),
Si(2)–O(3) = 1.654(4), Si(2)–O(4) = 1.652(4);
O(1)–Si(1)–O(2) = 110.6(2), O(3)–Si(2)–O(4) =
109.9(2). C = 1.179(9) and
1.213(9), Si(1)–O(1) = 1.635(4), Si(1)–O(2) = 1.646(4),
Si(2)–O(3) = 1.654(4), Si(2)–O(4) = 1.652(4);
O(1)–Si(1)–O(2) = 110.6(2), O(3)–Si(2)–O(4) =
109.9(2). | ||
We have now demonstrated that ring-closing alkyne metathesis with our ‘instant’ catalyst system provides a convenient route to novel alkyne-containing macrocycles. Functionalized rings should also be accessible via this methodology as we have already shown that the mixtures of Mo(CO)6 and 4-chlorophenol are metathesis-active in the presence of a variety of different functional groups.8,9
Acknowledgements
This work was generously supported by the NSF (CHE 9814118, PI Bunz) and the Research Corporation (PI Bunz).References
- R. R. Schrock, Polyhedron, 1995, 14, 3177 CrossRef CAS.
- R. H. Grubbs and S. Chang, Tetrahedron, 1998, 54, 4413 and references therein. Search PubMed.
- R. H. Grubbs, S. J. Miller and G. C. Fu, Acc. Chem. Res., 1995, 28, 446 CrossRef CAS.
- M. Schuster and S. Blechert, Angew. Chem., Int. Ed. Engl., 1997, 36, 2036 CrossRef; A. Fürstner, Top. Catal., 1997, 4, 285 Search PubMed.
- R. Gleiter and R. Merger, in Modern Acetylene Chemistry, ed. P. J. Stang and F. Diederich, VCH, Weinheim, 1995, ch. 8; Search PubMed; L. T. Scott and M. J. Cooney, in Modern Acetylene Chemistry, ed. P. J. Stang and F. Diederich, VCH, Weinheim, 1995, ch. 9. Search PubMed.
- U. H. F. Bunz and L. Kloppenburg, Angew. Chem., Int. Ed., 1999, 38, 478 CrossRef CAS.
- A. Fürstner and G. Seidel, Angew. Chem., Int. Ed., 1998, 37, 1734 CrossRef CAS.
- R. R. Schrock, D. N. Clark, J. Sancho, J. H. Wengrovius and S. F. Pedersen, Organometallics, 1982, 1, 1645 CrossRef CAS; X.-I. Zhang and G. C. Bazan, Macromolecules, 1994, 27, 4627 CrossRef CAS.
- L. Kloppenburg, D. Song and U. H. F. Bunz, J. Am. Chem. Soc., 1998, 120, 7973 CrossRef CAS; L. Kloppenburg, D. Jones and U. H. F. Bunz, Macromolecules, 1999, 32, 4194 CrossRef CAS.
- N. G. Pschirer and U. H. F. Bunz, Tetrahedron Lett., 1999, 40, 2481 CrossRef CAS.
- P. J. Stang and B. Olenyuk, Acc. Chem. Res., 1997, 30, 502 CrossRef CAS; P. J. Stang, Chem. Eur. J., 1998, 4, 19 CrossRef CAS; B. Olenyuk, A. Fechenkötter and P. J. Stang, J. Chem. Soc., Dalton Trans., 1998, 1707 RSC; B. Olenyuk, A. J. A. Whiteford, A. Fechenkötter and P. J. Stang, Nature, 1999, 398, 796 CrossRef CAS.
- For similar, conjugated silicon containing macrocycles, see F. Q. Liu, G. Harder and T. D. Tilley, J. Am. Chem. Soc., 1998, 120, 3271; Search PubMed; S. S. H. Mao, F. Q. Liu and T. D. Tilley, J. Am. Chem. Soc., 1998, 120, 1193 Search PubMed.
- M. Altmann and U. H. F. Bunz, Angew. Chem., Int. Ed. Engl., 1995, 34, 569 CrossRef CAS.
Footnotes |
| † Sample cyclization: 2 (1.00 g, 2.66 mmol), Mo(CO)6 (0.66 g, 0.27 mmol), 4-chlorophenol (0.206 g, 1.60 mmol) and 1,2-dichlorobenzene (100 ml) were held at 140 °C under a steady stream of N2 for 17 h. The solution was allowed to cool, then dissolved in hexanes and washed with dilute acid and base. Separation of the resulting products was achieved by chromatography over silica gel (Merck silica gel 60, 40–63 μm particle size; eluent 3∶1 hexanes–CH2Cl2−). Selected data for 3: δH(400 MHz, CDCl3) 7.31 (d, J 8.6, 12H), 6.71 (d, J 8.6, 12H), 1.30 (m, J 7.1, 6H), 1.16 (d, J 7.1, 36H); δC(400 MHz, CDCl3) 154.1, 132.7, 119.9, 116.8, 88.2, 17.2, 12.7. For 4: δH(400 MHz, CDCl3) 7.35 (d, J 8.8, 16H), 6.88 (d, J 8.8, 16H), 1.40 (m, 8H), 1.09 (d, J 7.3, 48H); δH(400 MHz, CDCl3) 154.4, 132.9, 119.7, 116.8, 88.1, 17.2, 12.7. |
‡ Crystal data: for
3: Si3O6C60H68,
triclinic, M = 936.12 g cm−3, space group =
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (No. 2). T = 293 K, Mo-Kα, a
= 23.76(2), b = 24.07(3), c = 18.7192(5) Å, α
= 92.0(1), β = 96.25(7), γ = 93.70(9)°, Z = 6,
D = 1.083 g cm−3, μ = 0.13
cm−1, 15889 measured reflections, 7703 independent
reflections, R (Rw) = 0.091 (0.126), hydrogen
atoms calculated, not refined. For 4:
Si4O8C80H176·2(C6
H14), triclinic, M = 1378.60, Space group =
P (No. 2). T = 293 K, Mo-Kα, a
= 23.76(2), b = 24.07(3), c = 18.7192(5) Å, α
= 92.0(1), β = 96.25(7), γ = 93.70(9)°, Z = 6,
D = 1.083 g cm−3, μ = 0.13
cm−1, 15889 measured reflections, 7703 independent
reflections, R (Rw) = 0.091 (0.126), hydrogen
atoms calculated, not refined. For 4:
Si4O8C80H176·2(C6
H14), triclinic, M = 1378.60, Space group =
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (No. 2), T = 293 K, Mo-Kα, a
= 13.977(8), b = 14.25(1), c = 12.144(7) Å, α
= 106.29(5), β = 108.21(5), γ = 69.68(5)°, Z = 1,
D = 1.083 g cm−3, μ = 0.12
cm−1, 4534 measured reflections, 2740 independent
reflections, R (Rw) = 0.0599 (0.0814).
Hydrogen atoms calculated, not refined. CCDC 182/1494. See
http://www.rsc.org/suppdata/cc/a9/a908638b/ for crystallographic data in
.cif format. (No. 2), T = 293 K, Mo-Kα, a
= 13.977(8), b = 14.25(1), c = 12.144(7) Å, α
= 106.29(5), β = 108.21(5), γ = 69.68(5)°, Z = 1,
D = 1.083 g cm−3, μ = 0.12
cm−1, 4534 measured reflections, 2740 independent
reflections, R (Rw) = 0.0599 (0.0814).
Hydrogen atoms calculated, not refined. CCDC 182/1494. See
http://www.rsc.org/suppdata/cc/a9/a908638b/ for crystallographic data in
.cif format. |
| This journal is © The Royal Society of Chemistry 2000 |

