Formation of a benzenetrienoid resonance structure in a cyclophane containing a trinitrotriaminobenzene unit
J. Jens
Wolff
*,
Uwe
Seibold
and
Thomas
Oeser
Organisch-Chemisches Institut der Ruprecht-Karls-Universität
Heidelberg, Im Neuenheimer Feld 270, D-69120 Heidelberg, Germany.. E-mail: wolff@donar.oci.uni-heidelberg.de
First published on 11th January 2000
Abstract
The [63](1,5,9)triphenyleno(1,3,5)cyclophane 1 shows a trienoid Kekulé-type structure for the planar donor–acceptor substituted benzene part.
The deviation of small aromatic molecules like benzene and naphthalene from the usual equal bond lengths to bond length alternations has been the object of synthetic and theoretical investigations.1,2 The known X-ray structures with considerable bond length alternations reminiscent of the extremum of a Kekulé structure require a threefold incorporation into strained rings which shorten the incorporated bonds. This leads to almost single-bond character for the bonds not incorporated. At first glance, steric strain may not be the only way to ease the formation of such structures with a Kekulé bond length alternation: the complete symmetrical substitution with three electron donating and three accepting groups might also lead to it. In an extreme situation, the interaction between donors and acceptors could equally result in Kekulé-type structures with three groups of separated double bonds that bear one donor and one acceptor, each interacting only with each other. Thus, this type of bond length alternation might also occur within such a ring without great strain.
A closer look, however, shows disappointing results. For example, while all 1,3,5-trinitro-2,4,6-triamino-substituted benzene derivatives known3–5 do show bond length alternations, they are far from the threefold symmetry required for a Kekulé structure: the six-membered ring has a clear maximum of C2v or C2 symmetry. Almost all of the central rings of the molecules analyzed by X-ray adopt either a strongly nonplanar boat or a twist form. The same holds true for substitution with other donors like the hydroxy group or its anion. For these highly non-planar molecules, the shown quinoid and bis-trimethine cyanoid resonance forms are the only geometric structures observed (Scheme 1). Planar ones correspond to usual benzene derivatives with some substituent-dependent bond length alternation.
 | ||
| Scheme 1 | ||
We were therefore surprised when an X-ray structure† (Scheme 2) was obtained of an intermediate 3 in the construction of bis-hexaaminobenzene derivatives6 used by us for the study of intramolecular electron transfer.7 Both aromatic rings are close to planar.8 While this would not be very surprising for most benzene derivatives, it has never been observed before for trinitrotriaminobenzenes, which are only close to planar in the hydrogen-bonded solid state structure of trinitrotriaminobenzene itself; even with only two amino groups and one alkylamino group, some folding of the ring is observed.4 In addition, the donor–acceptor substituted ring shows C3 symmetry and the bond lengths within the ring are 1.399(4) and 1.416(4) Å. Thus, the clear formation of a benzene ring with a Kekulé structure can be observed which lacks significant steric strain but has symmetrical donor–acceptor substitution. The higher C–C bond length occurs between the nitro and the amino groups that are linked by a weak intramolecular hydrogen bond (2.29 Å). Some interaction is also visible to the other direction, between the other oxygen of the nitro group and a proton of the methylene group next to the nitrogen (2.27 Å).9 The oxygen of the nitro group which is engaged in intramolecular hydrogen bonding with the amino group also shows intermolecular contact to the NH of the hexaazatriphenylene (2.34 Å). Both possible enantiomeric forms are present in the crystal, related to each other by inversion symmetry.
 | ||
| Scheme 2 | ||
A closer inspection provides a reason for the unexpected planarity. Since the bridges between the two rings need to keep approximately the same distance between them, they have to adopt comparable conformations. The hexaaminobenzene derivative part of 3 is expected to exist with a stable planar conformation for its central benzene ring.7,10,11 Then, the second, donor–acceptor substituted ring can adopt neither boat nor twist forms, because the distance of the chains to the first ring would become quite different (Scheme 3). These conformations are thus unlikely to be observed. While equality of the distances would be possible in a chair conformation, it is not truly compatible with the electronic requirements of the push–pull interaction between the substituents and is thus rarely found even for non-polar benzene derivatives with strong steric interactions.12 The only possible conformation for the usually highly distorted six-membered ring is therefore a planar one. While in this conformation the donor–acceptor interactions are quite reduced in comparison with known structures, they are nevertheless still present. Therefore a moderate, but definitely existent Kekulé distortion can be detected.
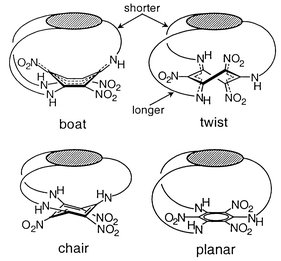 | ||
| Scheme 3 | ||
Acknowledgements
Support by the German Research Council (‘DFG’, Wo495/3-1 and Heisenberg fellowship for J. J. W.) is acknowledged. We would like to thank Mrs U. Wiesinger for assistance in the structural determination and also one of the reviewers for valuable comments.References
- F. Cozzi, F. Ponzini, R. Annunziata, M. Cinquini and J. S. Siegel, Angew. Chem., 1995, 107, 1092; Angew. Chem., Int. Ed. Engl., 1995, 34, 1019. Search PubMed.
- K. P. C. Vollhardt and D. L. Mohler, The Phenylenes: Synthesis, Properties, and Reactivity, in Advances in Strain in Organic Chemistry, Vol. 5, ed. B. Halton, JAI, Greenwich, 1996. Search PubMed.
- J. J. Wolff, Structures of Benzene Derivatives with Symmetrical Substitution by Three Donor and Three Acceptor Groups, in Advances in Strained and Interesting Organic Molecules, Vol. 7, ed. B. Halton, JAI, Stamford, 1999. Search PubMed.
- J. J. Wolff, F. Gredel, D. Hillenbrand and H. Irngartinger, Liebigs Ann. Chem., 1996, 1175 Search PubMed.
- K. K. Baldridge and J. S. Siegel, J. Am. Chem. Soc., 1993, 115, 10782 CrossRef CAS.
- Synthesis of 3: The synthesis of starting material will be described elsewhere. To a suspension of 1·3 HCl (235 mg, 0.360 mmol) in dry CH2Cl2, a mixture of Hünig’s base (282 mg, 2.18 mmol) and 1,3,5-trichloro-2,4,6-trinitrobenzene 2 (ref. 13) (114 mg, 0.360 mmol) in CH2Cl2 (100 ml) was added within 3 h. The mixture was stirred overnight and then, after addition of four drops of AcOH, filtered over silica gel (CH2Cl2–MeOH = 25/1). The solvents were removed and the chromatography was repeated. The cyclophane 3 was obtained as a yellow powder (18 mg, 0.024 mmol, 7%), decomp. >230 °C. Crystals for X-ray analysis were obtained from diffusion of pentane into a solution in CH2Cl2. δH(300 MHz, DMSO-d6) 1.08 (m, 6H), 1.26 (m, 6 H), 1.42 (m, 6H), 2.76 (m, 6H), 3.12 (m, 3H), 3.19 (m, 3H), 3.41 (d, J 17.1, 3H), 3.50 (d, J 16.9, 3H), 9.09 and 9.12 (br s, together 6H); δC(75.5 MHz, DMSO-d6) 23.30, 24.82, 27.38, 45.67, 53.53, 56.21, 114.82, 115.07, 126.57, 147.40, 168.97 [FAB-MS: calc. for C33H42N12O9: 750.32, found 750.48 (15, M+); C33H42N12O9 + Na+: 773.31, found: 773.48 (3, M + Na+)]..
- J. J. Wolff, A. Zietsch, H. Irngartinger and T. Oeser, Angew. Chem., 1997, 108, 637; Angew. Chem., Int. Ed. Engl., 1997, 36, 621. Search PubMed.
- The sum of absolute torsional angles within the donor-substituted six-membered ring is 19°, and 15° in the donor–acceptor substituted one..
- C–H···O interaction (see, e.g.: G. R. Desiraju, Acc. Chem. Res., 1996, 29, 441) could influence which type of bond is lengthened, and which one shortened. While the distance between NO and the second methylene group is fairly large (2.55 Å for C–H···O, 3.45 for C···O), it is smaller for the contact between the non-hydrogen-bonded oxygen and the first methylene group (2.27 and 2.99 Å). Since the nitrogen of the amino group will draw electron density from this carbon atom, it will also give a slightly more electron-deficient hydrogen more engaged in the dipolar interaction. AM1 or PM3 calculations indeed give a bond length alternation (0.026 vs. 0.017 Å) for a tris(diethylamino)trinitrobenzene with torsional angles derived from the structure of 3, which decreases for the corresponding trimethyl derivative (0.014 Å), and vanishes for the corresponding triamino derivative..
- J. Thomaides, P. Maslak and R. Breslow, J. Am. Chem. Soc., 1988, 110, 3970 CrossRef CAS.
- J. M. Chance, B. Kahr, A. B. Buda, J. P. Toscano and K. Mislow, J. Org. Chem., 1988, 53, 3226 CrossRef CAS.
- E.g.: H. Sakurai, K. Ebata, C. Kabuto and A. Sekiguchi, J. Am. Chem. Soc., 1990, 112, 1799..
- P. Engelbertz (Inv.), Chemische Fabrik Griesheim, D.R.P. 767510, 1936, Chem. Abstr., 1955, 49, 14803d; Search PubMed; M. E. Hill and F. Taylor Jr., J. Org. Chem., 1960, 25, 1037 Search PubMed.
Footnote |
| † Crystal data for 3: Orange hexagonal plates, C33H42N12O9·3H2 O·0.5 CH2Cl2 (750.3 + 96.5), T = 298 K, numerical absorption correction (μ = 0.16 mm–1). Trigonal space group P3 with a = b = 15.267(3), c = 10.921(6) Å, Z = 2; solution by direct methods with SHELXS-97, refinement with SHELXL-97. 3941 reflections, 3549 unique, 1715 observed [I > 2ς(I)]. N–H refined isotropically, remaining H calculated. R = 0.067, Rw = 0.187, GOF = 0.98. CCDC 182/1497. See http://www.rsc.org/suppdata/cc/a9/a908200j/ for crystallographic data in .cif format. |
| This journal is © The Royal Society of Chemistry 2000 |
