A rigid 1,3,5-phenylene-based metallodendrimer containing a ruthenium(II) bis(terpyridyl) complex
Mutsumi Kimura*, Tetsuo Shiba, Tsuyoshi Muto, Kenji Hanabusa and Hirofusa Shirai
Department of Functional Polymer Science, Faculty of Textile Science and Technology, Shinshu University, Ueda, Nagano, 386-8567, Japan.. E-mail: mkimura@giptc.shinshu-u.ac.jp
First published on UnassignedUnassigned6th January 2000
Abstract
A new 1,3,5-phenylene-based metallodendrimer containing a ruthenium bis(terpyridyl) complex was prepared and characterized; the rigid dendritic branches are found to affect the electrochemical properties of the redox-active core.
Dendrimers are well defined, highly branched macromolecules constructed from an interior core with a regular array of branch units.1 To investigate the influence of dendritic structures on electrochemical, photochemical and catalytic properties, dendrimers containing functional units have been designed and synthesized.2 The dendritic structures influence the microenvironment around the cores and modify their properties.3 Polypyridyl ruthenium(II) complexes ([Ru(bpy)3]2+ and [Ru(tpy)2]2+) are widely used for the construction of dendrimers because of their unique electrochemical and photochemical properties.4 However, these dendrimers have been prepared by attaching flexible dendron units around metal complexes. Rigid dendrimers have been prepared using phenylene, phenylacetylene and phenylenevinylene units as repeating units in the dendritic structures.5 The construction of a well defined rigid dendritic structure around a redox-active metal complex unit may result in interesting electrochemical and optical changes.
Herein, we report the first rigid metallodendrimer containing [Ru(tpy)2]2+ in the interior core. The synthesis of the new 1,3,5-phenylene-based dendritic ligand 3 was performed using the methodology developed by Miller et al. (Scheme 1).6 The terpyridyl core 1 was obtained from 3,5-dibromobenzaldehyde and 1-(2-pyridylcarbonylmethyl)pyridinium iodide according to the literature.7 The dendron 2 was prepared in a stepwise manner through a Suzuki coupling reaction between an arylboronic acid and 3,5-dibromo-1-(trimethylsilyl)benzene.8 Finally, the coupling of 2 to 1 using Pd(PPh3)4 as a catalyst proceeded smoothly to give the dendritic ligand 3 in 70% yield. This new dendritic ligand was characterized by 1H NMR and matrix-assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometric techniques. MALDI-TOF mass spectra of 3 gave the correct molecular peak ([M + H+] m/z = 1823). Fig. 1 shows the 1H NMR spectrum of 3 in CDCl3 which provides structural information. The 1H NMR spectrum in the aromatic region of 3 is very simple and readily assignable and indicates the successive generation of a highly symmetric layered structure.
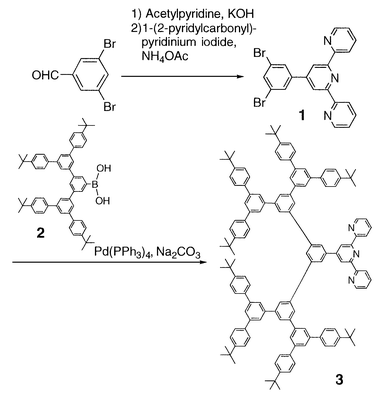 | ||
| Scheme 1 | ||
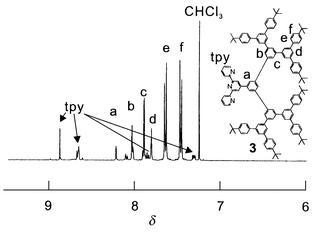 | ||
| Fig. 1 1H NMR spectrum of 3 in CDCl3. | ||
Treatment of 3 with RuCl3·3H2O generated the desired metallodendrimer [Ru(3)2]2+ (Scheme 2) which was purified by column chromatography after anion exchange with hexafluorophosphate ion. The presence of tert-butyl groups at the exterior positions of the dendrimer completely encapsulates the Ru(tpy)2 core and enhances the hydrophobicity with metallodendrimer [Ru(3)2]2+ being highly soluble in a variety of organic solvents such as alkanes, but not in polar solvents such as acetonitrile and alcohols. By contrast, the low molecular weight complex bis(4′-(p-tolyl)-2,2′∶6′,2″-terpyri dyl)ruthen- ium(II) complex ([Ru(tpy-phMe)2]2+) only showed solubility in polar solvents. The Homogeneity of [Ru(3)2]2+ was demonstrated by GPC analyses and MALDI-TOF mass spectrometry. Examination by GPC analysis showed that [Ru(3)2]2+ has a sharp and symmetrical elution peak with a polydispersity (Mw/Mn) of <1.01. The MALDI-TOF mass spectrum of [Ru(3)2]2+ gave the correct mass at m/z 3891 for the [M − PF6] peak. Fig. 2 shows the absorption spectra of 3 and [Ru(3)2]2+, with absorption maxima (λmax) and molar absorption coefficients (ε) collected in Table 1. The spectrum of [Ru(3)2]2+ in the visible region showed a characteristic MLCT transition at 490 nm, indicating the formation of a [Ru(tpy)2]2+ core.10 The molecular coefficient for the MLCT band of [Ru(3)2]2+ was almost the same as that of [Ru(tpy-phMe)2]2+. Moreover, the absorbance at 260 nm of [Ru(3)2]2+ was approximately double that of the dendritic ligand 3. As revealed by a computer generated ball-and-stick model, the dendritic ligand 3 is a hemispherical structure with a radius of ca. 1.5 nm (Scheme 2). The metallodendrimer [Ru(3)2]2+ is thus formed by metal-mediated assembly through the formation of a metal complex between two ligands possessing well defined hemispherical dendritic structures and one Ru2+ ion.
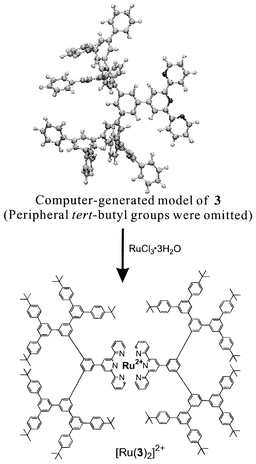 | ||
| Scheme 2 | ||
![UV–VIS absorption spectra of 3 and
[Ru(3)2]2+ in CH2Cl2.
([3], [Ru(3)2]2+ = 1.0 μM).](/image/article/2000/CC/a908500i/a908500i-f2.gif) | ||
| Fig. 2 UV–VIS absorption spectra of 3 and [Ru(3)2]2+ in CH2Cl2. ([3], [Ru(3)2]2+ = 1.0 μM). | ||
| m/za | Absorptionb λmax/nm (ε/M−1 cm−1) | E1/2c/V vs. SCE | ΔEc/ mV | |
|---|---|---|---|---|
| a m/z Value determined by MALDI-TOF experiments.b CH2Cl2 solution.c From cyclic voltammetry in CH2Cl2 solution, 100 mV s−1 scan rate, Bu4NPF6 supporting electrolyte, Pt electrode, Ag/AgCl reference. E1/2 is defined as the average of the two voltages at the current maximum/minimum of the redox waves. ΔE is defined as the difference of the peak voltages for the reduction and return oxidation waves. | ||||
| [Ru(3)2]2+ | 3891 | 490 (25000) | +1.25 | 180 |
| [M − PF6−] | 260 (653000) | −1.28 | 120 | |
| −1.43 | 125 | |||
| [Ru(tpy-phMe)2]2+ | 490 (28900) | +1.20 | 76 | |
| −1.24 | 71 | |||
| −1.46 | 83 | |||
The influence of the rigid dendritic branches on the redox properties of the redox-active [Ru(tpy)2]2+ core was studied by cyclic voltammetry in CH2Cl2 using Bu4NPF6 (0.1 M) as supporting electrolyte (Table 1). The metallodendrimer [Ru(3)2]2+ exhibited one oxidation (E1/2 = +1.25 V vs. SCE) and two reduction processes (E1/2 = −1.28 and −1.43 V vs. SCE). The average peak potentials (E1/2) for these processes were almost the same as those of the non-dendiritic [Ru(tpy-phMe)2]2+ complex.4a,9 However, [Ru(3)2]2+ showed a large voltage difference between the current maxima of the reduction and return oxidation wave (ΔE), indicative of slower electron transfer than for the non-dendritic complex. This observation was similar to the electrochemical results of a flexible metallodendrimer containing redox-active core subunits.3,4 The rigid dendritic branches around the [Ru(tpy)2]2+ core lead to a distance of 1.5 nm between the core and the electrode surface. The remoteness of the redox-active core from the electrode surface was found to hinder the electron transfer processes.
In summary, we have reported the construction of a new rigid metallodendrimer, in which the metal complex is encapsulated in a precise position within the rigid dendritic macromolecule. Further photochemical investigations of the rigid metallodendrimer are currently in progress.
Acknowledgements
This research was supported in part by a Grant-in-Aid for COE Research ‘Advanced Fiber/Textile Science and Technology’ (#10CE2003) and Scientific Research (#11450366) from the Ministry of Education, Science, Sports and Culture of Japan.References
- D. A. Tomalia and H. Dupont Durst, Top. Corr. Chem., 1993, 165, 193 Search PubMed; G. R. Newkome and C. N. Moorefield, Comprehensive Supramolecular Chemistry, Pergamon, 1996, vol. 10 pp. 777–832; Search PubMed; J. M. J. Fréchet, Science, 1994, 263, 1710 Search PubMed; F. Zeng and S. C. Zimmerman, Chem. Rev., 1997, 97, 1681 CrossRef CAS.
- C. Gorman, Adv. Mater., 1998, 10, 295 and references therein; Search PubMed; M. Fischer and F. Vögtle, Angew. Chem., Int. Ed., 1999, 38, 884 and references therein. Search PubMed.
- P. J. Dandliker, F. Diederich, M. Gross, C. B. Knobler, A. Louati and E. M. Sandford, Angew. Chem., Int. Ed. Engl., 1994, 33, 1739 CrossRef; P. J. Dandliker, F. Diederich, J.-P. Gisselbrecht, A. Louati and M. Gross, Angew. Chem., Int. Ed. Engl., 1995, 34, 2725 CrossRef CAS; H.-F. Chow, I. Y.-K. Chan, D. T. W. Chan and R. W. M. Kwok, Chem. Eur. J., 1996, 9, 1085; C. B. Gorman, B. L. Parkhurst, W. Y. Su and K.-Y. Chen, J. Am. Chem. Soc., 1997, 119, 1141 CrossRef CAS; C. M. Cardona and A. E. Kaifer, J. Am. Chem. Soc., 1998, 120, 4023 CrossRef CAS.
- (a) G. R. Newkome, R. Güther, C. N. Moorefield, F. Cardullo, L. Echegoyen, E. Pérez-Cordero and H. Luftmenn, Angew. Chem., Int. Ed. Engl., 1995, 34, 2023 CrossRef CAS; (b) J. Issberner, F. Vögtle, L. De Cola and V. Balzani, Chem. Eur. J., 1997, 3, 706 CrossRef CAS; (c) F. Vögtle, M. Plevoets, M. Nieger, G. C. Azzellini, A. Credi, L. De Cola, V. De Marchis, M. Venturi and V. Balzani, J. Am. Chem. Soc., 1999, 121, 6290 CrossRef; (d) V. Balzani, S. Campagna, G. Denti, A. Juris, S. Serroni and M. Venturi, Acc. Chem. Res., 1998, 31, 26 CrossRef CAS; and references therein..
- T. M. Miller and T. X. Neenan, Chem. Mater., 1990, 23, 34; Z. Xu, M. Kahr, K. L. Walker, C. L. Wilkins and J. S. Moore, J. Am. Chem. Soc., 1994, 116, 4537 CrossRef CAS; C. Devadoss, P. Bharathi and J. S. Moore, J. Am. Chem. Soc., 1996, 118, 9635 CrossRef CAS; D. J. Pesak, J. S. Moore and T. E. Wheat, Macromolecules, 1997, 30, 6467 CrossRef CAS; C. Devadoss, P. Bharathi and J. S. Moore, Macromolecules, 1998, 31, 8091 CrossRef CAS; H. Meier and M. Lehmann, Angew. Chem., Int. Ed., 1998, 37, 643 CrossRef CAS; F. Morgenroth, A. J. Berresheim, M. Wagner and K. Müllen, Chem. Commun., 1998, 1139 RSC.
- T. M. Miller, T. X. Neenan, R. Zayas and H. E. Bair, J. Am. Chem. Soc., 1992, 114, 1018 CrossRef CAS.
- F. Kröhnke and K. F. Gross, Chem. Ber., 1959, 92, 22 Search PubMed; J. P. Collin, S. Guillerez and J. P. Sauvage, J. Chem. Soc., Chem. Commun., 1989, 776 RSC; K. Hanabusa, A. Nakamura, T. Koyama and H. Shirai, Macromol. Chem., 1992, 193, 1309 Search PubMed.
- N. Miyata, T. Yanagai and A. Suzuki, Synth. Commun., 1981, 11, 513 CrossRef CAS.
- E. C. Constable and M. D. Ward, J. Chem. Soc., Dalton Trans., 1990, 14 Search PubMed; J.-P. Collin, S. Guillerez, J.-P. Sauvage, F. Barigelletti, L. De Cola, L. Flamigni and V. Balzani, Inorg. Chem., 1991, 30, 4230 CrossRef CAS; J. C. Chambron, C. Coudret and J.-P. Sauvage, New J. Chem., 1992, 16, 361 Search PubMed; F. Barigelletti, L. Flamigni, V. Balzani, J.-P. Collin, A. Sour, E. C. Constable and A. M. W. Cargill Thompson, J. Chem. Soc., Chem. Commun., 1993, 942 RSC; F. Barigelletti, L. Flamigni, V. Balzani, J.-P. Collin, J.-P. Sauvage, A. Sour, E. C. Constable and A. M. W. Cargill Thompson, J. Am. Chem. Soc., 1994, 116, 7692 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2000 |
