The first ester complexes of bismuth(III) using thiolate anchored bifunctional ligands
Glen G.
Briand
,
Neil
Burford
* and
T. Stanley
Cameron
*
Department of Chemistry, Dalhousie University, Halifax, Nova Scotia B3H 4J3, Canada.. E-mail: burford@is.da.ca
First published on 6th January 2000
Abstract
The isolation and characterization of bismuth complexes involving ester functionalities on bifunctional ligands demonstrates the use of thiolates as anchors for weaker donors and in the context of the medicinal relevance of bismuth compounds, offers the opportunity to study the interaction of all biorelevant functional groups with bismuth.
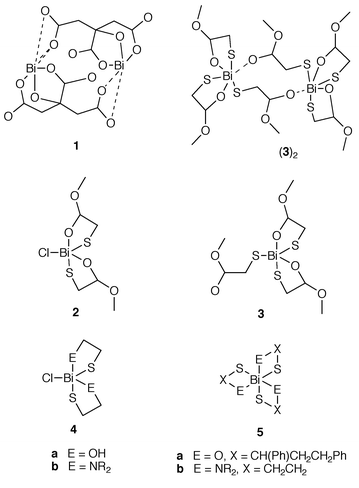
The developing coordination chemistry of bismuth is hindered by the facile hydrolysis of most bismuth–element bonds to give the bismuthyl unit (BiO+), which involves essentially quantitative precipitation.1 Consequently, complexes of weakly donating functionalities can only be isolated in the absence of moisture and many conventional types of ligand have not yet been observed on bismuth. The high thermal and hydrolytic stability of the sulfur–bismuth bond2 has enabled synthetic control using bifunctional ligands involving a thiolate anchor,3 which offer the additional advantage of satisfying the high coordinative capacity of the bismuth center and thereby inhibit intermolecular interactions and coordination polymerisation. As a result, the complexes exhibit relatively high solubility allowing for crystallization and structural and spectroscopic characterization. We have exploited these realizations to prepare the first examples of ester complexes of bismuth, despite the relatively low basicity of the ester (carbonyl) (pKb 21.5; cf. OH pKb 16.5, NR2 pKb 9.2, CO2− pKb 9.2) functionality. The significance of these new complexes lies in their relationship to the extensive and medicinally relevant carboxylate chemistry of bismuth. In this context, one of the new ester complexes adopts a pendant donor intermolecular arrangement that is reminiscent of the ubiquitous dimer structure 1 observed for the most extensively studied colloidal bismuth subcitrate (CBS).1,4–7
Reactions of potassium (methylester)methanethiolate with bismuth(III) chloride in 95% ethanol at the appropriate stoichiometry give bis[(methylester)methanethiolato]bismuth(III) chloride 2 (2∶1) and tris[(methylester)methanethiolato]bismuth(III) 3 (3∶1), respectively.†‡ Reaction of (methylester)methanethiol with bismuth(III) chloride also give 2, which was identified by its distinctive Raman spectrum as the dominant product, independent of reaction stoichiometry (4∶1, 3∶1, 2∶1).
Compound 2 adopts a one-dimensional polymeric array in the solid state (Fig. 1), with hepta-coordination for bismuth imposed by four equatorially disposed sulfur centers, two oxygen centers (carbonyl) and one chlorine center. The long and essentially equivalent Bi–S distances result from the strong trans influence8 induced by intermolecular Bi–S contacts [S(1)-Bi–S(2a) 154.5(2) and S(2)–Bi–S(1a) 170.3(1)°]. Although a molecular unit represented by drawing 2 is indistinguishable in the polymeric solid state structure, the analogy with the more molecular hydroxy/thio 4a9 and amino/thio 4b3,9,10 derivatives is important. We attribute the more polymeric structure of 2 to the restrictions imposed by the backbone sp2 hybridized carbonyl carbon center and the consequential chelate ring strain.
![Crystallographic view of polymeric arrangement of
[Bi(SCH2CO2- Me)2Cl] 2. Thermal
ellipsoids are drawn to 50% probability.](/image/article/2000/CC/a907094j/a907094j-f1.gif) | ||
| Fig. 1 Crystallographic view of polymeric arrangement of [Bi(SCH2CO2- Me)2Cl] 2. Thermal ellipsoids are drawn to 50% probability. | ||
The structure of the tris(esterthiolato)bismuth complex (Fig. 2) may, at first glance, be viewed as two tris-chelated bismuth centers 3, as observed for the keto/thiolate 5a11 and aminothiolate complexes 5b.3 However, closer inspection of the Bi–S bond distances [i.e. Bi⋯S(2a), 3.331(2) is substantially longer than Bi–S(2), 2.608(2), Table 1] reveals that one of the ligands clearly functions as an internuclear (Bi⋯Bi) bridge, rather than a chelating ligand, imposing a distinct dimer structure (3)2. In comparison with the polymeric structure of 2, substitution of the chloride for a third thiolate is manifested in the dislocation of the ⋯S2BiS2Bi⋯ chain with consequential enhancement (shortening) of the three facial thiolate interactions (Table 1).
![Crystallographic view of the dimeric arrangement of
[Bi(SCH2CO2Me)3] 3. Thermal
ellipsoids are drawn to 50% probability.](/image/article/2000/CC/a907094j/a907094j-f2.gif) | ||
| Fig. 2 Crystallographic view of the dimeric arrangement of [Bi(SCH2CO2Me)3] 3. Thermal ellipsoids are drawn to 50% probability. | ||
| 2 | 3 | 4a 9 | 5a 11 | CBS12,16–19 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a Average bond distances for two unique molecules. | |||||||||
| Bi–O(1) | 2.68(2) | Bi–O(1) | 2.807(5) | Bi–O | 2.80(1) | Bi–Oa | 2.575(11) | ||
| Bi–O(3a) | 2.77(2) | Bi–O(6) | 2.861(5) | 2.86(1) | 2.614 | ||||
| 2.537(10) | |||||||||
| Bi···O(4a) | 3.071(7) | Bi···O | 2.4–2.6 | ||||||
| Bi–S(1) | 2.849(7) | Bi–S(1) | 2.568(2) | Bi–S | 2.595(3) | Bi–Sa | 2.724(3) | ||
| Bi–S(2) | 2.884(6) | Bi–S(2) | 2.608(2) | 2.558(4) | 2.581(4) | ||||
| Bi–S(1a) | 2.963(7) | Bi–S(3) | 2.574(2) | 2.659(5) | |||||
| Bi–S(2a) | 2.861(9) | ||||||||
| Bi···S(2a) | 3.331(2) | Bi···S | 3.124(4) | Bi···S | 3.494(5) | ||||
| Bi–Cl(1) | 2.535(6) | Bi–Cl | 2.589(3) | 3.551(5) | |||||
| Bi···Cl | 3.488(4) | ||||||||
The dimeric structure (3)2 imposed by the pendant ester is analogous to that observed for CBS 1.12 In contrast, the methyl groups preclude the interdimer interactions observed in the carboxylate complexes, resulting in a relatively simple molecular structure and highlighting the ester functionality as an important stepping stone to understanding the carboxylate chemistry of bismuth.
The identification and isolation of the first ester complexes of bismuth is demonstrative of the synthetic value of the thiolate as an anchor for weaker donors at the other terminus of bifunctional ligands. In the compelling quest to understand the bioactivity of bismuth compounds,13–15 we now have the opportunity to study the interaction of all biorelevant functional groups with bismuth. For example, we are currently developing synthetic procedures towards bifunctional thiolate/amide [C(O)NR2] ligand complexes, which represent an alternative derivatization of the carboxylate functionality and for which we anticipate an intermediate number of intermolecular interactions between those of the carboxylate and ester complexes.
References
- G. G. Briand and N. Burford, Adv. Inorg. Chem., 1999in press. Search PubMed.
- L. Agocs, N. Burford, T. S. Cameron, J. M. Curtis, J. F. Richardson, K. N. Robertson and G. B. Yhard, J. Am. Chem. Soc., 1996, 118, 3225 CrossRef CAS.
- G. G. Briand, N. Burford, T. S. Cameron and W. Kwiatkowski, J. Am. Chem. Soc., 1998, 120, 11374. Search PubMed.
- G. G. Briand and N. Burford, Chem. Rev., 1999, 99, 2601 CrossRef CAS.
- G. F. Baxter, Pharm. J., 1989, 243, 805 Search PubMed.
- G. F. Baxter, Chem. Br., 1992, 445 Search PubMed.
- H. Sun, H. Li and P. J. Sadler, Chem. Ber./Recueil, 1997, 130, 669 Search PubMed; Coord. Chem. Rev., 1999, 185–186, 689. Search PubMed.
- F. J. Carmalt and N. C. Norman, in Chemistry of Arsenic, Antimony and Bismuth, ed. N. C. Norman, Blackie Academic and Professional, London, 1998, pp. 1–38. Search PubMed.
- L. Agocs, G. G. Briand, N. Burford, T. S. Cameron, W. Kwiatkowski and K. N. Robertson, Inorg. Chem., 1997, 36, 2855 CrossRef CAS.
- W. A. Herrmann, P. Kiprof, W. Scherer and L. Pajdla, Chem. Ber., 1992, 125, 2657 CAS.
- A. K. Mishra, V. D. Gupta, G. Linti and H. Nöth, Polyhedron, 1992, 11, 1219 CrossRef CAS.
- E. Asato, K. Katsura, M. Mikuriya, T. Fujii and J. Reedijk, Inorg. Chem., 1993, 32, 5322 CrossRef CAS.
- W. A. Herrmann, E. Herdtweck and L. Pajdla, Chem. Ber., 1993, 126, 895 CAS.
- U. Dittes, E. Vogel and B. K. Keppler, Coord. Chem. Rev., 1997, 163, 345 CrossRef CAS.
- S. P. Summers, K. A. Abboud, S. R. Farrah and G. J. Palenik, Inorg. Chem., 1994, 33, 88 CrossRef CAS.
- E. Asato, K. Katsura, M. Mikuriya, U. Turpeinen, I. Mutikainen and J. Reedijk, Inorg. Chem., 1995, 34, 2447 CrossRef CAS.
- W. A. Herrmann, E. Herdtweck and L. Pajdla, Inorg. Chem., 1991, 30, 2579 CrossRef CAS.
- W. A. Herrmann, E. Herdtweck and L. Pajdla, Z. Kristallogr., 1992, 198, 257 Search PubMed.
- P. J. Barrie, M. I. Djuran, M. A. Mazid, M. McPartlin and P. J. Sadler, J. Chem. Soc., Dalton Trans., 1996, 2417 RSC.
Footnotes |
| † [Bi(SCH2CO2Me)2Cl] 2: BiCl3 (1.67 g, 5.28 mmol) added to methylthioglycolate (1.12 g, 10.6 mmol) in 95% ethanol (150 mL) was allowed to stir overnight. The product was removed by suction filtration and recrystallized from DMF under vacuum (yellow needles). Yield 1.10 g (46%); mp 128 °C; Anal. Calc.: C, 15.85; H, 2.22%. Found: C, 16.08; H, 2.26%; IR(cm−1): 555w, 681w, 770w, 874s, 886w, 986m, 994m, 1161m, 1206m, 1296m, 1318m, 1676s, 1707s; Raman (cm−1): 95vs, 117s(sh), 147s, 187s, 227vs, 260vs, 350m, 399w, 561w, 686w, 768w, 888m, 984w, 1181w, 1204w, 1325w, 1376w, 1392w, 1428w, 1673w, 2903s, 2940m, 2959m, 3044w; 1H NMR (dmso−d6): δ 3.62, 4.69; 13C NMR (dmso-d6): δ 29.6, 52.3, 176.3; APCI−MS (rel. % intensity): 313(2), 349(100), 419(32).[Bi(SCH2CO2Me)3] 3: BiCl3 (1.65 g, 5.23 mmol) added to methylthioglycolate (1.67 g, 15.7 mmol) and KOH (0.88 g, 16 mmol) in 95% ethanol (150 mL) under N2 was allowed to stir overnight. The solution was filtered and concentrated by rotary evaporation. Yellow needles of 1 appeared after 2 h at 4 °C, collected after 1 day. Yield 0.71 g (26%); mp 65 °C; Anal. Calc.: C, 20.61; H, 2.88%. Found: C, 20.71; H, 2.84%; IR(cm−1): 569m, 583m, 681w, 711m, 772w, 864m, 880w, 889m, 899w, 909w, 992s, 1021w, 1141m, 1204s, 1287s, 1308s, 1397m, 1434s, 1558w, 1579w, 1605w, 1623w, 1691m, 1735m; Raman (cm−1): 95vs, 146m, 195m, 221m, 266vs, 292vs, 334m, 402w, 580w, 714w, 769w, 885w, 908w, 990w, 1151w, 1181w, 1296w, 1397w, 1435w, 1696w, 2886s, 2956m, 2976m, 2988m, 3034w; 1H NMR (dmso-d6): δ 3.63, 4.46; 13C NMR (dmso-d6): δ 29.4, 52.3, 175.8; APCI-MS (rel. intensity): 313(4), 419(100). |
‡ Crystal
data. for 2:
C6H10BiClO4S2, M =
454.70, monoclinic, space group Pa (no. 7), light yellow needles,
a = 8.092(2), b = 9.13(1), c = 8.128(3) Å,
β = 102.90(2)°, V = 585.5(6) Å3,
Dc = 2.556 g cm−3, Z = 2,
T = 23.0 °C, R = 0.036, Rw =
0.027, GOF 1.57. For 3:
C9H15BiO6S3, M 524.36,
triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), yellow needles,
a = 9.340(1), b = 11.553(3), c = 8.168(1)
Å, α = 109.18(2), β = 92.98(2), γ = 103.38(2)°,
V = 798.4(3) Å3, Dc = 1.993 g
cm−3, Z = 2, T = 23.0 °C, R
= 0.0297, Rw = 0.0293, GOF 1.60.All measurements were made on a Rigaku AFC5R diffractometer with
graphite monochromated Mo-Kα radiation (λ = 0.71069 Å)
and a 12 kW rotating anode generator. The structures were solved by direct
methods (SHELXL97) and refined by full-matrix least least squares on
F using 608 (2) and 3230 (3) reflections with
I > 3.00ς(I). A final difference-Fourier map
yielded ρ(max.) = 0.72 e Å−3 and ρ(min.) =
−0.69 e Å−3 for 2 and ρ(max.) =
1.26 e Å−3 and ρ(min.) = −0.80 e
Å−3 for 3. CCDC 182/1488. (no. 2), yellow needles,
a = 9.340(1), b = 11.553(3), c = 8.168(1)
Å, α = 109.18(2), β = 92.98(2), γ = 103.38(2)°,
V = 798.4(3) Å3, Dc = 1.993 g
cm−3, Z = 2, T = 23.0 °C, R
= 0.0297, Rw = 0.0293, GOF 1.60.All measurements were made on a Rigaku AFC5R diffractometer with
graphite monochromated Mo-Kα radiation (λ = 0.71069 Å)
and a 12 kW rotating anode generator. The structures were solved by direct
methods (SHELXL97) and refined by full-matrix least least squares on
F using 608 (2) and 3230 (3) reflections with
I > 3.00ς(I). A final difference-Fourier map
yielded ρ(max.) = 0.72 e Å−3 and ρ(min.) =
−0.69 e Å−3 for 2 and ρ(max.) =
1.26 e Å−3 and ρ(min.) = −0.80 e
Å−3 for 3. CCDC 182/1488. |
| This journal is © The Royal Society of Chemistry 2000 |