Electrostatic interactions between positively charged porphyrins and nucleotides or amides: buffer-dependent dramatic changes of binding affinities and modes†
Mallena Sirish and Hans-Jörg Schneider*
FR 11.2 Organische Chemie der, Universität des Saarlandes, D-66041, Saarbrücken, Germany.. E-mail: ch12hs@rz.uni-sb.de
First published on UnassignedUnassigned24th December 1999
Abstract
The association strength of positively charged porphyrins with anionic species can be orders of magnitude higher than reported in the literature, depending on the chosen buffer; the buffer also determines the dominating interaction mechanism, leading to stronger stacking contributions at higher salt concentrations; even electroneutral amides show a hitherto not recognized interaction between the negatively charged carbonyl oxygen and the positively charged π-surface of the porphyrin system.
Porphyrins with pyridinium substituents (R) have been used for some time as partners in supramolecular complexes, in particular with nucleotides,1 as well as ligands for DNA.2 As major binding contributions, electrostatic and stacking interactions have been identified.1,3 In the context of recent studies with a new porphyrin-based peptide receptor4 we have measured associations with the underlying tetrapyridinium framework R under different conditions and have found large affinity changes to anionic species such as nucleotides. The results indicate at the same time strong changes in the binding modes, supported by the observed complexation-induced NMR shifts.
The binding constants were determined as reported earlier;4 the titrations showed isosbestic points, in line with the fitting to a 1∶1 equilibrium. It was secured that there was no pH change during titration; the very small change of the anyway very low ionic strength has a negligible influence on the association equilibria.5 Measurements with amides like A1–A5 showed changes of ΔA in the Soret band absorption similar to those observed earlier with a related receptor and peptides.4 Association constants around 5000 M−1 with diamides A1–A4 and over 10000 M−1 with the triamide A5 reveal that even eletroneutral molecules can strongly interact with the positive charges in the extended aromatic receptor moiety. Experiments with corresponding diesters E (n = 1–3) and also α,ω-alkane diols with R showed in contrast to the other ligands no appreciable ΔA in the UV spectra, indicating that hydrophobic or lipophilic interactions cannot be a major binding force. These observations are only in line with additive interactions between the cationic porphyrin skeleton and the carbonyl oxygen atoms, which are known to bear a substantial negative charge in amides, in contrast to e.g. in esters where we cannot detect binding.
If such interactions with amide oxygens already lead to such high affinities, larger numbers should be expected with fully charged anions. In consequence, the association constants are expected to be substantially lowered by anions present in concentrations below the molarity of the amide type ligands. We therefore decided to re-eaxamine the affinity of the often used tetrapyridinium porphyrin R towards different anions, with and without the commonly used buffers.1 Indeed we find that the binding constants are larger by one to two orders of magnitude than those reported in the literature (Table 1), if one measures them not in the presence of added buffer anions; this is illustrated with the selected isotherms in Fig. 1. Even with simple halides we find rather large log K values (Table 1).
| No buffer | 0.1 M buffer | 0.3 M buffer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands | logK | Δλ/nm | ΔA | logK | Δλ/nm | ΔA | logK | Δλ/nm | ΔA |
| a Measured by UV–visible titration of R with nucleotides in water in the absence and in the presence of 0.1 and 0.3 M phosphate buffer (pH 7.9 ± 0.2) at 25 °C. Titrations were carried out by adding concentrated stock solutions of nucleotides ([nucleotide] = 10 mM) containing ca. 2 μM of porphyrin to an equally concentrated solutions of porphyrin in a 10 mm cuvet. Error limits: logK ± 5% | |||||||||
| b . K value can not be determined accurately as the ΔA value is negligible. | |||||||||
| Adenosine | 4.54 | 3 | 0.11 | 2.64 | 5 | 0.39 | 2.79 | 6 | 0.35 |
| AMP2− | 4.52 | 9 | 0.38 | 3.40 | 5 | 0.20 | 2.87 | 5 | 0.31 |
| ADP3− | 4.95 | 10 | 0.38 | 3.40 | 7 | 0.10 | 3.14 | 7 | 0.33 |
| ATP4− | 5.45 | 13 | 0.37 | 3.34 | 8 | 0.27 | 3.55 | 7 | 0.23 |
| dGMP2− | 4.42 | 7 | 0.42 | 2.88 | 4 | 0.18 | 2.54 | 5 | 0.29 |
| Thymidine | 4.80 | 3 | 0.18 | 2.40 | 3 | 0.27 | 2.27 | 5 | 0.28 |
| TMP2− | 4.42 | 6 | 0.27 | 2.56 | 3 | 0.21 | 2.33 | 3 | 0.25 |
| dUMP2− | 4.09 | 6 | 0.32 | 3.98 | 2 | 0.07 | 2.44 | 2 | 0.30 |
| dCMP2− | 4.02 | 5 | 0.29 | 4.03 | 1 | 0.06 | 2.38 | 3 | 0.24 |
| H3PO42− | 4.73 | 2 | 0.27 | 3.31 | 1 | 0.05 | b | 0 | <0.02 |
| NH4Cl | 4.55 | 2 | 0.05 | ||||||
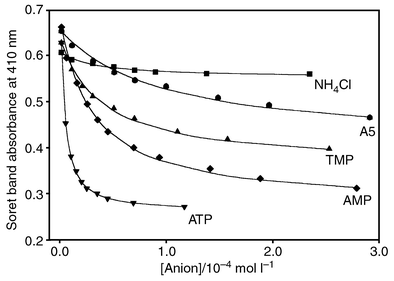 | ||
| Fig. 1 Non-linear least square fit of UV–visible titration curves for receptor R with selected anions. Conditions: AMP, ATP and TMP without buffer (see Table 1); A5 in water at pH 6.9 ± 0.2; NH4Cl in water without buffer at pH 7.9 ± 0.2. | ||
Association of nucleotides with tetrapyridinium porphyrin is thus much stronger than known before; it increases as expected with the charges in the range AMP<ADP<ATP, and falls off steeply with higher buffer concentrations (Table 1). Noticeably, the distinct base selectivity observed, in the older work between purine and pyrimidine bases with R,1 is almost absent at low salt concentrations, and appears again with higher buffer concentrations. Obviously, in the absence of competing buffer anions the electrostatic interactions between the nucleotide phosphate and the cationic receptor dominate so strongly that stacking interactions play a minor role. On the other hand, with decreased ion pairing at higher buffer concentrations the lipophilic interactions gain in energetic importance, as the salt bridges do not ‘draw away’ as strongly the nucleobase from contacts to the porphyrin surface. That the nucleotide indeed stacks to the porphyrin moiety is established, by the significant shielding observed both at the nucleobase and the ribose protons.
In conclusion, the results show that the buffers used in host–guest complexation studies can significantly alter the dominating non-covalent interaction mechanisms, and thereby both the affinity and the selectivity seen in such systems. Moreover, permanent charges in a large receptor surface such as the tetrapyridinium porphyrin can even in water also lead to significant binding with the partial negative charges of electroneutral species such as amides.
Acknowledgements
Our work is supported by the Deutsche Forschungsgemeinschaft, Bonn, and the Fonds der Chemischen Industrie, Frankfurt. M. S. thanks the A. v. Humboldt foundation for a stipend.References
- R. F. Pasternack, E. J. Gibbs, A. Gaudemer, A. Antebi, S. Bassner, L. De Poy, D. H. Turner, A. Williams, F. Laplace, M. H. Lansard, C. Merienne and M. Perree-Fauvet, J. Am. Chem. Soc., 1985, 107, 8179 CrossRef CAS; Y. Kuroda, H. Hatakeyama, N. Inakoshi and H. Ogoshi, Tetrahedron Lett., 1993, 51, 8285; B. L. Iverson, K. Shreder, V. Kral, P. Sansom, V. Lynch and J. L. Sessler, J. Am. Chem. Soc., 1996, 118, 1608 CrossRef CAS; J. L. Sessler and A. Andrievsky, Chem. Eur. J., 1998, 4, 159 CrossRef CAS.
- B. Meunier, Chem. Rev., 1992, 92, 1411 CrossRef CAS; and references cited therein; ; H. J. Schneider and M. Wang, J. Org. Chem., 1994, 59, 7473 Search PubMed; P. Bigey, G. Pratviel and B. Meunier, J. Chem. Soc., Chem. Commun., 1995, 181 CrossRef CAS; N. E. Mukundan, G. Petho, D. W. Dixon and L. G. Marzilli, Inorg. Chem., 1995, 34, 3677 RSC; G. Pratviel, J. Bernadou and B. Meunier, Angew. Chem., 1995, 107, 819 CrossRef CAS; Angew Chem., Int. Ed. Engl., 1995, 34, 746; ; D. W. Dixon and V. Steullet, J. Inorg. Biochem., 1998, 69, 25.
- H.-J. Schneider, Chem. Soc. Rev., 1994, 22, 227 RSC; and references cited therein; ; H.-J. Schneider and M. Wang, J. Org. Chem., 1994, 59, 7464 Search PubMed.
- M. Sirish and H.-J. Schneider, Chem. Commun., 1999, 907 RSC; M. Sirish, F. Eblinger and H.-J. Schneider, Advances in Supramolecular Chemistry, ed. G. W. Gokel, JAI, Stamford, 1999, vol. 6, in the press. Search PubMed.
- See Md. Alamgir Hossain and H.-J. Schneider, Chem. Eur. J., 1999, 5, 1284. Search PubMed.
Footnote |
| † Supramolecular Chemistry, Part 93; Part 92: V. A. Palyulin, S. V. Emets, V. A. Chertkov, C. Kasper and H.-J. Schneider, Eur. J. Org. Chem., 1999, in the press. |
| This journal is © The Royal Society of Chemistry 2000 |