Metal catalysed addition of B–H and N–H bonds to aminopropyl vinyl ethers
Christopher M. Vogels, Paul G. Hayes, Michael P. Shaver and Stephen A. Westcott*
Department of Chemistry, Mount Allison University, Sackville, NB E4L 1G8, Canada.. E-mail: swestcott@mta.ca
First published on UnassignedUnassigned7th January 2000
Abstract
The rhodium catalysed addition of pinacolborane to aminopropyl vinyl ethers gave selective formation of the corresponding alkenylboronate esters while palladium or platinum complexes catalysed the hydroamination of aminopropyl vinyl ether to give tetrahydro-2-methyl-1,3-oxazine.
The catalytic addition of B–H bonds to unsaturated carbon–carbon bonds using 1,3,2-benzodioxaborole (catecholborane) is of significant importance in organic synthesis.1 Indeed, the use of a metal catalyst can give organoboronate ester products with stereo-, chemo- and regio-selectivities complementary or opposite to those obtained from the corresponding uncatalysed variants. As part of our ongoing study of generating novel aminoboron compounds,2 we have examined the catalysed addition of B–H bonds to aminopropyl vinyl ethers (R2NCH2CH2CH2OCH
![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH
2) and report our initial findings herein.
CH
2) and report our initial findings herein.We have found that addition of catecholborane to commercially available aminopropyl vinyl ether 1a and dialkylated derivatives 1b–e† using 5 mol% of RhCl(PPh3)3 gave a number of products derived from an initial dehydrogenative borylation pathway. Formation of products derived from such pathways are well precedented2a,3–5 and presumably occur via initial oxidative addition of the catecholborane B–H bond to the rhodium centre. This step is followed by insertion of the alkene into the Rh–B bond with subsequent ß–H abstraction to give dihydrogen and alkenylboronate esters.2a Further reaction of alkenylboronate esters formed in this study with catecholborane or dihydrogen occurs rapidly, as evidenced by the disappearance of the alkenyl peaks in the 1H NMR spectra, to give a number of borated products. Product distributions were further complicated by the formation of Lewis acid–base adducts between the amine moiety and either unreacted catecholborane or the borated products. Although a number of rhodium complexes‡ were investigated as possible catalysts for this reaction, all gave several boron-containing products.
Pinacolborane (HBpin, pin = 1,2-O2C2Me4), a less reactive hydroborating agent than catecholborane, was used in an attempt to improve selectivities for this reaction. Interestingly, reactions using HBpin and 5 mol% of RhCl(PPh3)36 at 65 °C gave the corresponding alkenylboronate esters 2a–e (Scheme 1) as the only new boron-containing species.§ Similar reactivities were observed using other rhodium catalysts.‡ Although metal catalysed alkenylboronate ester formation using catecholborane is well established,3–5 analogous products derived from such a pathway using pinacolborane are much less common and only occur in low yields.6
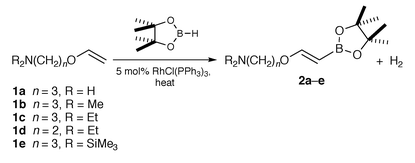 | ||
| Scheme 1 | ||
Unlike hydroborations of aminoalkenes using 9-BBN
(9-borabicyclo[3.3.1]nonane),7 protection of
the amine hydrogens is not necessary when R = H, as HBpin reacts rapidly
with 1a to form the intermediate
(pinB)NHCH2CH2CH2OCH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH2
with loss of dihydrogen. The weak Lewis-acidic nature of HBpin also
precludes the formation of adducts, which are observed in reactions with
catecholborane.
CH2
with loss of dihydrogen. The weak Lewis-acidic nature of HBpin also
precludes the formation of adducts, which are observed in reactions with
catecholborane.
The formation of alkenylboronate esters in these reactions is intriguing
as previous studies on the addition of B–H bonds to vinyl ethers
using BH3 have shown exclusive formation of
anti-Markovnikov hydroboration products.8 We have also found that addition of HBpin to ethyl
vinyl ether under similar catalytic conditions gave the corresponding
borated product CH3CH2OCH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CHBpin, suggesting
that the amine group is not responsible for the formation of
alkenylboronate products in reactions with aminopropyl vinyl ethers.9 The directive effects exerted by heteroatoms on
the hydroboration of substituted vinyl derivatives has been reported
elsewhere.8,10–12
CHBpin, suggesting
that the amine group is not responsible for the formation of
alkenylboronate products in reactions with aminopropyl vinyl ethers.9 The directive effects exerted by heteroatoms on
the hydroboration of substituted vinyl derivatives has been reported
elsewhere.8,10–12
The methodology described above provides a rapid and efficient means of generating novel air-stable alkenylboronate esters, which have remarkable synthetic utility in Suzuki–Miyaura cross-coupling reactions.13 Vinyl ether derivatives are also versatile synthetic intermediates in polymer chemistry.14 Further work is currently being conducted on the role the catalyst and the effect the heteroatom α to the vinyl group plays on selectivities in these reactions.
Interestingly, while some palladium and platinum complexes are known to be efficient hydroboration catalysts,1 we have found that ‘hydroboration reactions’ using 1a and catalytic amounts of palladium and platinum compounds gave products derived from addition of the corresponding borane to tetrahydro-2-methyl-1,3-oxazine 3. Formation of cyclic 315 presumably proceeds via an intramolecular hydroamination of the starting aminopropyl vinyl ether (Scheme 2). In fact, in the absence of a hydroborating agent, a number of platinum and palladium complexes effectively catalyse (using 5 mol% of metal) this reaction with high turnover numbers (Table 1).¶ The palladium catalysed intramolecular hydroamination reaction is generally believed to occur via initial coordination of the alkene to the metal centre, which acts to polarize the alkene moiety and thereby making it more susceptible to nucleophilic attack by the amine.16 In this study, subsequent addition of the N–H bond to the alkene moiety results in selective formation of the six-membered ring product.
 | ||
| Scheme 2 | ||
While palladium iodide (Table 1, entry 1) is an active catalyst precursor for this hydroamination reaction, reduced activities (entry 7) were observed in reactions utilizing the corresponding chloride salt. Higher turnover numbers were achieved, however, using the cyclooctene metal chloride dimers (entry 4), presumably owing to their increased solubility in common organic solvents.2b Reactions using preformed bis-amine complexes (for instance PdI2L2, where L = 3, entry 2) also gave oxazoline formation in high turnover numbers suggesting that these complexes may be resting states in the catalytic cycle. Attempts to affect the intermolecular hydroamination of ethyl vinyl ether and propylamine using these catalysts proved unsuccessful. Likewise, no reaction was observed when 2-(1-cyclohexenyl)ethylamine was treated with these metal complexes, suggesting that the ether group in aminopropyl vinyl ether may also facilitate in activating the alkene towards nucleophilic attack. Studies of an asymmetric variant of the hydroamination chemistry, as well as expanding the scope of this reaction, are currently under investigation.
Acknowledgements
Thanks are gratefully extended to Drs Terri Bright, Giuliano Muccin and Sabine Weiguny (BASF) for the generous gift of aminopropyl vinyl ether and diethylaminoethyl vinyl ether, Dr R. Tom Baker (LANL) for helpful discussions, the Natural Sciences and Engineering Research Council of Canada, Mount Allison University, and Johnson Matthey Ltd. for the palladium and platinum salts.References
- For an excellent review on hydroborations catalysed by transition metal complexes, see: I. Beletskaya and A. Pelter, Tetrahedron, 1997, 53, 4957. Search PubMed.
- (a) T. M. Cameron, R. T. Baker and S. A. Westcott, Chem. Commun., 1998, 2395 RSC; (b) C. M. Vogels, H. L. Wellwood, K. Biradha, M. J. Zaworotko and S. A. Westcott, Can. J. Chem., 1999, 77, 1196 CrossRef CAS.
- K. Burgess, W. A. van der Donk, S. A. Westcott, T. B. Marder, R. T. Baker and J. C. Calabrese, J. Am. Chem. Soc., 1992, 114, 9350 CrossRef CAS.
- S. A. Westcott, T. B. Marder and R. T. Baker, Organometallics, 1993, 12, 975 CrossRef CAS.
- J. M. Brown and G. C. Lloyd-Jones, J. Am. Chem. Soc., 1994, 116, 866 CrossRef CAS.
- S. Pereira and M. Srebnik, Tetrahedron Lett., 1996, 37, 3283 CrossRef CAS.
- G. W. Kabalka, N.-S. Li and R. D. Pace, Synth. Commun., 1995, 25, 2135 CrossRef CAS.
- H. C. Brown and R. L. Sharp, J. Am. Chem. Soc., 1968, 90, 2915 CrossRef CAS.
- R. T. Baker, R. D. Broene, C. M. Vogels and S. A. Westcott, unpublished data..
- D. J. Pasto and J. Hickman, J. Am. Chem. Soc., 1967, 89, 5608 CrossRef CAS.
- For other directive effects in hydroborations, see: B. Singaram, C. T. Goralski and G. B. Fisher, J. Org. Chem., 1991, 56, 5691. Search PubMed.
- A. H. Hoveyda, D. A. Evans and G. C. Fu, Chem. Rev., 1993, 93, 1307 CrossRef CAS.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS.
- P. Kohli, A. B. Scranton and G. J. Blanchard, Macromolecules, 1998, 31, 5681 CrossRef CAS.
- H. Booth and R. U. Lemieux, Can. J. Chem., 1971, 49, 777 CAS.
- T. E. Muller and M. Beller, Chem. Rev., 1998, 98, 675 CrossRef; Y. Li and T. J. Marks, J. Am. Chem. Soc., 1998, 120, 1757 CrossRef CAS; P. J. Walsh, A. M. Baranger and R. G. Bergman, J. Am. Chem. Soc., 1992, 114, 1708 CrossRef CAS; M. R. Gagné, C. L. Stern and T. J. Marks, J. Am. Chem. Soc., 1992, 114, 275 CrossRef CAS; L. S. Hegedus, Angew. Chem., Int. Ed. Engl., 1988, 27, 1113 CrossRef; M. S. Driver and J. F. Hartwig, Organometallics, 1997, 16, 5706 CrossRef.
Footnotes |
| † Dialkylated aminopropyl vinyl ethers were prepared by addition of 2.2 equivalents of BunLi to 1a at −78 °C in THF under an atmosphere of dinitrogen. After 2 h, 2 equivalents of the corresponding alkyl halide were added dropwise at 0 °C. Removal of solvent and extraction with hexane, followed by distillation, gave the desired products. Spectroscopic NMR data (CDCl3): 1b (15% yield): 1H δ 6.37 (q, J 5 Hz, 1H), 4.08 (d, J 16 Hz, 1H), 3.87 (d, J 5 Hz, 1H), 3.62 (t, J 5 Hz, 2H), 2.29 (t, J 5 Hz, 2H), 2.10 (s, 6H), 1.74 (m, 2H); 13C{1H} δ 151.8, 86.4, 66.3, 56.4, 45.5, 27.3. 1c (10% yield): 1H δ 6.41 (q, J 5 Hz, 1H), 4.11 (d, J 16 Hz, 1H), 3.89 (d, J 5 Hz, 1H), 3.65 (t, J 5 Hz, 2H), 2.47 (overlapping m, 6H), 1.76 (m, 2H), 0.95 (t, J 5 Hz, 6H); 13C{1H} δ 152.0, 86.3, 66.6, 49.5, 47.1, 26.9, 11.9. 1e (27% yield): 1H δ 6.50 (q, J 5 Hz, 1H), 4.18 (d, J 16 Hz, 1H), 3.99 (d, J 5 Hz, 1H), 3.65 (t, J 5 Hz, 2H), 2.92 (t, J 8 Hz, 2H), 1.76 (m, 2H), 0.07 (s, 18H); 13C{1H} δ 152.1, 86.2, 65.9, 42.6, 34.8, 2.24. |
| ‡ Other complexes examined include [RhCl(cod)]2 (cod = cycloocta-1,5-diene), RhH(PPh3)4, RhH(CO)(PPh3)3, [RhCl(coe)2]2 (coe = cis-cyclooctene) and [RhCl(coe)2]2/2PPh3. |
| § The following procedure was used for the dehydrogenative borylation of aminopropyl vinyl ethers. Pinacolborane (190 mg, 1.5 mmol) was added to a solution of aminopropyl vinyl ether (1 mmol) and catalyst (5 mol%) in 10 mL of THF. The mixture was heated to reflux for 3 h under an atmosphere of dinitrogen whereupon solvent was removed and the reaction mixture analyzed by multinuclear NMR spectroscopy. Selected NMR data (CDCl3): 2a: 1H δ 7.07 (d, J 16 Hz, 1H), 4.43 (d, J 16 Hz, 1H), 3.80 (m, 2H), 3.47 (m, 2H), 1.81 (m, 2H), 1.22 (s, 12H); 11B{1H} δ 34.0; 13C{1H} δ 162.2, 88.0 (br, C–B), 82.2, 68.0, 40.5, 32.2, 23.9. 2b: 1H δ 6.99 (d, J 16 Hz, 1H), 4.41 (d, J 16 Hz, 1H), 3.81 (t, J 5 Hz, 2H), 2.99 (m, 2H), 2.64 (s, 6H), 2.08 (br m, 2H), 1.24 (s, 12H); 11B{1H} δ 31.0;13C{1H} δ 162.5, 87.7 (br, C-B), 82.7, 67.9, 51.6, 46.3, 26.2, 24.7.2c: 1H δ 7.04 (d, J 16 Hz, 1H), 4.43 (d, J 16 Hz, 1H), 3.81 (t, J 5 Hz, 2H), 3.42 (t, J 5 Hz, 2H), 2.51 (overlapping m, 6H), 1.24 (s, 12H), 1.03 (t, J 5 Hz, 6H); 11B{1H}, δ 31.4; 13C{1H} δ 163.0, 87.3 (br, C–B), 82.6, 67.0, 48.9, 46.5, 26.2, 24.5, 11.0. 2d: 1H δ 7.09 (d, J16 Hz, 1H), 4.46 (d, J 16H z, 1H), 3.87 (t, J 8 Hz, 2H), 2.76 (t, J 5 Hz, 2H), 2.61 (q, J 8 Hz, 4H), 1.25 (s, 12H), 1.05 (t, J 8 Hz, 6H); 11B{1H} δ 31.5; 13C{1H} δ 162.6, 87.3 (br, C–B), 82.9, 66.0, 51.0, 47.5, 24.8, 10.6. 2e: 1H δ 7.06 (d, J 16 Hz, 1H), 4.41 (d, J 16 Hz, 1H), 3.68 (t, J 8 Hz, 2H), 2.87 (m, 2H), 1.69 (m, 2H), 1.23 (s, 12H), 0.07 (s, 18H); 11B{1H} δ 32.0; 13C{1H} δ 163.4, 87.3 (br, C–B), 82.8, 66.6, 42.4, 34.7, 24.9, 2.2 |
| ¶ In a typical reaction, a known amount of aminopropyl vinyl ether was added to a stirred solution or suspension of catalyst (5 mol%) in 10 mL of toluene. The reaction mixture was allowed to stir for an additional 6 h upon which solvent was removed and the reaction analyzed by 1H NMR spectroscopy.15 |
| This journal is © The Royal Society of Chemistry 2000 |
