Vanadium [dicyanoperfluorostilbene]2·yTHF: a molecule-based magnet with Tc ≈ 205 K†
Jeffrey P. Fitzgeralda, Bharat B. Kaulb and Gordon T. Yee*b
aDepartment of Chemistry, United States Naval Academy, Annapolis, MD 21402, USA
bDepartment of Chemistry and Biochemistry, University of Colorado, Boulder, CO 80309, USA.. E-mail: yeeg@colorado.edu
First published on UnassignedUnassigned7th January 2000
Abstract
A new radical anionic bridging ligand, derived from the in situ reduction of α,α′-dicyanoperfluorostilbene, is reported to support ferrimagnetic ordering below 205 K in a three-dimensional vanadium-based coordination polymer.
In 1991, Miller and coworkers reported that the reaction of V(C6H6)2 and tetracyanoethylene (TCNE), in dichloromethane yields an air-sensitive solid that is magnetically ordered at room temperature.1 This compound, formulated as V(TCNE)2·0.5CH2Cl2 based on elemental analysis, represented the first example of ferrimagnetism above room temperature in a molecule-based system. The structure is presumed to be a three-dimensional coordination polymer consisting of solvent-ligated V2+ cations bridged by TCNE·1− anions, though only limited structural information has been obtained.2 One factor perhaps contributing to the amorphous nature of the solid is that, in principle, TCNE may bridge up to four metal centers, resulting in a degree of structural randomness.3 Miller and coworkers have subsequently studied the effects of coordinating solvent2 and improved on the synthesis, replacing V(C6H6)2 with the more readily available precursor, V(CO)6.4 In recent work, they have shown that MI2·xMeCN salts, (M = Mn, Fe, Co or Ni), are viable sources of building blocks for analogous M(TCNE)2 magnets in which Tc ranges from 44 to 121 K.5
Despite this progress, no organic acceptor has been reported to substitute for TCNE in reactions with V(CO)6 or V(C6H6)2 to give a magnetically ordering compound.2 Although many candidates possess the requisite electrochemical properties, those that have been examined, including 7,7,8,8-tetracyanoquinodimethane (TCNQ), 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and tetrachloro-1,4-benzoquinone, react to yield paramagnetic solids, none of which show any signs of magnetic order.2 These results have left it unclear what special properties of TCNE, such as molecular size, shape, redox properties or the ability to coordinate more than two metal centers, are critical to achieving a magnetically ordered solid. The absence of another efficacious acceptor has hampered efforts to elucidate the detailed mechanism of magnetic coupling in V(TCNE)2 and to further develop this intriguing family of ferrimagnetic three-dimensional coordination polymers.
Here we report the discovery of a second magnetically ordering compound in the V(acceptor)2·y(solvent) family. Pursuing a strategy involving the design and synthesis of ethylenic acceptors related to TCNE,6 has led to the discovery that α,α′-dicyanoperfluorostilbene, (DCPFS), reacts with V(CO)6 in THF to give an air-sensitive, charge-transfer coordination polymer, V(DCPFS)2·yTHF, a compound that orders, apparently ferrimagnetically, with Tc ≈205 ± 5 K. Because many of the characteristics of DCPFS are so unlike those of TCNE, its examination helps to delineate the properties that are unimportant in the design of potential new radical anionic bridging ligands. The construction of coordination polymer magnets with radical anionic bridging ligands is an attractive approach because the versatility of organic chemistry can be exploited and because the compounds produced are inherently three-dimensional, which favors higher Tc values.
DCPFS was prepared by the oxidative dimerization of pentafluorophenylacetonitrile utilizing a modification of the method described by Cook and Linstead.7,8 Both cis and trans products are fully characterized, air-stable, crystalline, white solids. Using cyclic voltammetry, it was found that trans-DCPFS exhibits a quasi-reversible one-electron reduction at ca. −1.0 V vs. ferrocene.8 This value is 0.7 V more negative than the first reduction of TCNE, revealing that DCPFS is only a modest electron acceptor. It does not, for instance, oxidize decamethylmanganocene in CH2Cl2.
In spite of its relatively negative reduction potential, DCPFS reacts readily with V(CO)6. In a typical preparation, V(CO)6 in THF is treated with 2.2 equiv. of trans-DCPFS at room temp. in an inert atmosphere glove box.8 The color immediately changes from yellowish green to reddish purple, but in sharp contrast to reactions between V(CO)6 and TCNE, this reaction appears to remain homogeneous for at least several minutes after mixing of the two reactants. Over the course of 1 h, however, a small amount of dark precipitate deposits on the sides of the flask. This initial precipitate exhibits magnetic order, but at a relatively low Tc, perhaps because the degree of polymerization is still low. To obtain the higher Tc material, the solvent is removed under vacuum and the residue is triturated in Et2O for 15 min. The black solid is collected on a fritted glass filter and washed with Et2O until the filtrates run clear. Once precipitated, the solid cannot be redissolved in the original solvent.
Unfortunately, despite the greater solubility of incipient V(DCPFS)2·yTHF in organic solvents, we have not yet been able to crystallize it. Powder X-ray diffraction shows that the isolated compound is amorphous. IR spectrometry reveals little about the coordination environment except that there is no CO present.Elemental analysis of the product is variable, as was previously observed for the TCNE analog.4 However, the results on five independently prepared samples are roughly consistent with the formulation, V(DCPFS)2·yTHF, (y ≈ 2). The analyses were usually high in vanadium, a fact not too surprising given the method of isolation.
To support this proposed formulation, additional experiments have been performed in which the reactant ratios were varied. As expected, preparing the compound beginning with a 1∶1 ratio of acceptor to donor, resulted in a product with a much lower critical temperature, and in much lower yield. In contrast, the product obtained from the 4∶1 reactant ratio was magnetically equivalent to that obtained from the 2∶1 procedure and the quantity of isolated product was essentially the same.
A sample [henceforth abbreviated V(DCPFS)2] for magnetic studies was sealed under vacuum in a glass holder as previously described.9 The plot of gram magnetization, M, vs. temperature, T, measured in 2 G applied field is shown in Fig. 1. It clearly shows the onset of magnetic order below ca. 205 K. The continuous decrease in magnetization with increasing temperature is reminiscent of the behavior observed for the TCNE analog. The feature at ca. 120 K might be the result of a second magnetic phase transition or indicate the presence of a second magnetic phase. The plot of ac susceptibility for V(DCPFS)2 is also quite similar to that observed for V(TCNE)2.10 The data appear as a broad, featureless, frequency-independent maximum in χ′ with a small non-zero χ″ component.8
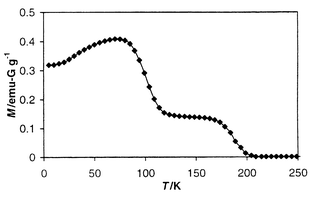 | ||
| Fig. 1 Plot of magnetization, M, vs. temperature, T, measured at 2 G. | ||
Like V(TCNE)2, V(DCPFS)2 is a soft ferromagnet. Fig. 2 shows the plot of gram-magnetization, M, vs. applied field, H, at 5 K. At this temperature, the amount of hysteresis is very small; the coercive field is <20 G. Gram-magnetization was plotted because of the uncertainty in the formula unit. However, assuming the stoichiometry V(DCPFS)2·2THF, one calculates the saturation magnetization to be ca. 7000 emu-G mol−1, roughly consistent with ferrimagnetic coupling of two spin 1/2 anions and one spin 3/2 cation per formula unit.
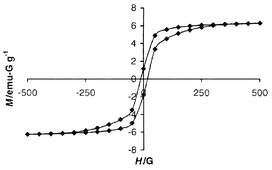 | ||
| Fig. 2 Plot of magnetization, M, vs. applied field, H, measured at 5 K. | ||
It is important to note that the observed magnetic properties are not due to an impurity phase: by cooling a sample to 77 K in a sealed round bottom flask, one can easily attract and move the entire sample with a Sm–Co permanent magnet. Further, while one might suspect the presence of a vanadium-based Prussian blue-type ferrimagnet via the reductive decomposition of DCPFS, that seems very unlikely given no such rationally designed phase has been reported that contains only vanadium and no other metal.12–14 Also, if such a compound did exist, it would have to possess ordered CN ligands around ordered mixed-valent V cations (no low-spin electron configurations exist for octahedral V in oxidation states two or higher) and these would have to arise from our one-pot synthesis.
Several modifications of the above standard procedure for preparing V(DCPFS)2 were found to result in samples with inferior magnetic properties. For instance, carrying out the reaction in CH2Cl2 instead of THF gives a material with Tc ≈ 100 K. This result is in sharp contrast with the corresponding reaction of V(CO)6 with TCNE for which CH2Cl2 is the solvent of choice and coordinating solvents such as THF give rise to products with lower Tc values.14 In THF, using V(C6H6)2 in place of V(CO)6 as a source of V0 results in an ordering phase, although the Tc is considerably lower (ca. 80 K). On the other hand, we have found that cis-DCPFS reacts with V(CO)6 to give a magnetically identical product to that obtained from trans-DCPFS. This result, coupled with the observation that the Et2O washes contain mixtures of cis- and trans-DCPFS, suggests that isomerization about the double bond occurs upon formation of the radical anion.
In conclusion, we have discovered V(DCPFS)2·yTHF to be a molecule-based magnet possessing a Tc of 205 K. Its magnetic behavior is quite reminiscent of the room temperature magnet, V(TCNE)2. It is unclear what sets TCNE and DCPFS apart from other organic acceptors in reactions with V(CO)6 and V(C6H6)2. At this point, we can comment more meaningfully on the differences between DCPFS and TCNE and their impact on the choice of future synthetic targets: 1 Redox properties; TCNE is a relatively good acceptor, but DCPFS is a poor acceptor. This means that many more weak acceptor bridging ligands need to be investigated for their ability to mediate exchange. 2 Number of N donor sites; TCNE has four, but DCPFS has only two. Thus, despite the fact that each DCPFS ligand can act as a bridge between only two V cations, a ratio of two acceptors per donor is sufficient to form a three-dimensionally ordered magnetic solid. 3 Molecular size and shape; planar TCNE is the smallest acceptor and DCPFS is one of the largest and probably not flat. This result can be interpreted to mean that steric repulsion does not play a significant role, provided the fumaronitrile unit is left intact. Thus, larger, more complex ligands can be envisioned and should be investigated.
Acknowledgements
We thank the National Science Foundation for support of this work and the National Institute of Standards and Technology for use of the SQUID magnetometers. We also acknowledge Mr Christopher M. Holloway for synthetic help and Mr Roger Sommer for electrochemistry experiments.References
- J. M. Manriquez, G. T. Yee, R. S. McLean, A. J. Epstein and J. S. Miller, Science, 1991, 252, 1415 CrossRef CAS
.
- J. S. Miller and A. J. Epstein, Chem. Commun., 1998, 1319 RSC
.
- W. Kaim and
M. Moscherosch,
Coord. Chem. Rev., 1994,
129, 157 and references
therein. Search PubMed
.
- J. Zhang,
J. S. Miller,
C. Vazquez,
P. Zhou,
W. B. Brinckerhoff and
A. J. Epstein, in
Molecule-Based Magnetic Materials, Theory, Techniques, and
Applications, ed. M. M. Turnbull, T. Sugimoto and L. K. Thompson,
ACS Symp. Ser., Washington DC,
1996, vol. 6444, pp.
311–318. Search PubMed
.
- J. Zhang, J. Ensling, V. Ksenofontov, P. Gütlich, A. J. Epstein and J. S. Miller, Angew. Chem., Int. Ed., 1998, 37, 657 CrossRef CAS
.
- B. B. Kaul, W. S. Durfee and G. T. Yee, J. Am. Chem. Soc., 1999, 121, 6862 CrossRef CAS
.
- A. H. Cook and
R. P. Linstead,
J. Chem. Soc., 1937,
929. Search PubMed
.
- See Electronic Supplementary Information (ESI) for complete experimental procedures and the plot of ac susceptibility [http://www.rsc.org/suppdata/cc/a9/a907535f/]..
- S. P. Sellers,
B. J. Korte,
J. P. Fitzgerald,
W. M. Reiff and
G. T. Yee, J.
Am. Chem. Soc., 1998, 120,
4662. Search PubMed
.
- B. G. Morin, P. Zhou, C. Hahm, A. J. Epstein and J. S. Miller, J. Appl. Phys., 1993, 73, 5648 CrossRef CAS
.
- W. R. Entley, C. R. Treadway and G. S. Girolami, Mol. Cryst. Liq. Cryst., 1995, 273, 153 Search PubMed
.
- S. M. Holmes and
G. S. Girolami,
J. Am. Chem. Soc., 1999,
121, 5593 and references
therein. Search PubMed
.
- O. Hatlevik, W. E. Buschmann, J. Zhang, J. L. Manson and J. S. Miller, Adv. Mater., 1999, 11, 914 CrossRef CAS
.
- P. Zhou, S. M. Long, J. S. Miller and A. J. Epstein, Phys. Lett. A, 1993, 181, 71 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: preparation and spectral data for α,α′-dicyanoperfluorostilbene. See http://www.rsc.org/suppdata/cc/a9/a907535f/ |
| This journal is © The Royal Society of Chemistry 2000 |