Synthesis, photoluminescent and electroluminescent behaviour of four-coordinate tetrahedral gold(I) complexes. X-Ray crystal structure of [Au(dppn)2]Cl†
Vivian
Wing-Wah Yam
*,
Chui-Ling
Chan
,
Sam
Wing-Kin Choi
,
Keith
Man-Chung Wong
,
Eddie
Chung-Chin Cheng
,
Sze-Chit
Yu
,
Po-King
Ng
,
Wai-Kin
Chan
and
Kung-Kai
Cheung
Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong SAR, P.R. China.. E-mail: wwyam@hku.hk
First published on 6th January 2000
Abstract
Two tetrahedral four-coordinate Au(I) complexes, [Au(4-R-dppn)2]PF6 (R = H or Me), have been synthesized and the crystal structure of [Au(dppn)2]Cl has been determined by X-ray crystallography; the complexes have been found to exhibit both photoluminescent and electroluminescent behaviour.
Unlike d10 Cu(I) and Ag(I) species in which complexes with coordination number greater than two are commonly observed, most Au(I) complexes adopt a two-coordinate linear geometry.1 Studies on four-coordinate Au(I) systems2,3 have mainly been confined to those of structural characterization, with a few examples on cytotoxic4a and antitumor activities.4b Despite the recent growing interest in the study of luminescent two-5 and three-coordinate6 Au(I) systems, there have been no reports on the luminescence behaviour of four-coordinate Au(I) complexes. Besides, with the growing interest in the design and construction of light-emitting diodes (LEDs)7 and the employment of metal complexes8 as the emissive layer in electroluminescence (EL) devices, the use of Au(I) complexes in such studies is rare8f,g despite the rich luminescence behaviour associated with a number of Au(I) systems.5,6,9
Here, we report the synthesis of two four-coordinate Au(I) complexes, [Au(4-R-dppn)2]X [dppn = 1,8-bis(diphenylphosphino)naphthalene; R = H, X = PF61a, Cl 1b; R = Me, X = PF62], and the X-ray crystal structure of 1b. The first report on the photoluminescent and electroluminescent properties of this tetrahedral four-coordinate Au(I) system is also described.
To the freshly reduced solution of Au(I), prepared in situ by treatment of K[AuCl4] (0.1 mmol) with an excess of 2,2′-thiodiethanol in methanol (20 ml), was added the diphosphine ligand, 4-R-dppn (0.2 mmol). The chloride salt of the product was isolated by slow evaporation of the solvent. Diffraction quality crystals of 1b were obtained by recrystallization from acetonitrile. Orange solids of 1a and 2 were collected after the metathesis reaction with NH4PF6 in methanol. Recrystallization from dichloromethane–diethyl ether afforded 1a and 2 as orange crystals. The identities of both have been confirmed by 1H NMR spectroscopy, IR, positive FAB-MS, and elemental analyses.† The X-ray crystal structure of 1b has also been determined.‡
Fig. 1 depicts the perspective view of the complex cation of 1b. The structure shows a Au(I) centre in a highly distorted tetrahedral coordination geometry, as required by the steric demand of the dppn ligand. The P–Au–P angle of 86.9(2)–133.3(2)° are comparable to other four-coordinate Au(I) diphosphine complexes such as [Au(dppe)2]+ [P–Au–P 85.4(1)–129.6(1)°]3c and [Au(pdma)2]+ [pdma = o-phenylenebis(dimethylarsine), P–Au–P 86.7–121.8°].3a The Au–P bonds of 2.379(6)–2.388(6) Å are slightly shorter than those of [Au(dppe)2]+ [2.389(3)–2.416(3) Å],3c but are longer than those of two-coordinate Au(I) phosphine complexes.5e,f,9
 | ||
| Fig. 1 Perspective drawing of the complex cation of 1b. | ||
The electronic absorption spectra of 1a and 2 are very
similar, and are dominated by an intense high energy absorption band at
ca. 300 nm with a weak low energy band at ca. 400 nm with
tails extending to ca. 500 nm. The photophysical data of
1a and 2 are collected in Table
1. With reference to previous spectroscopic work,9,10 the intense absorption at ca.
300 nm which closely resembles that found in the free ligand, is assigned
as an intraligand π→π*(naphthalene) transition while the lowest
energy absorption band is a σ→π*(naphthalene) transition. An
assignment of these low-energy absorption bands as d→p transitions,
similar to those suggested for three-coordinate d10 complexes of
Au(I)6b,c and
Pt(0)5a,11 is unlikely, given
the approximately tetrahedral AuP4 structure in which the
valence p orbitals are strongly σ antibonding. Such d→p
transitions are expected to occur at fairly high energy, for example, at
240 nm for [Au(dcpe)2]+ [dcpe =
1,2-bis(dicyclohexylphosphino)ethane].6b Excitation of solid samples of
1a and 2 gave intense orange emission both at room
temperature and 77 K. Room temperature luminescence in degassed
dichloromethane solution has also been observed. The observation of the
emission lifetime in the microsecond range is suggestive of an emission
origin associated with a spin-forbidden transition. No such emission was
observed in the related [Au(dcpe)2]+ complex, which
was reported to be non-emissive both in the solid state and in
solution.6b However, similar
emission bands were observed in a related two-coordinate
[Au2(dppn)(C![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) CR)2],9d in which an assignment of the emission
origin as derived from states of σ→π*(naphthalene) transition
is suggested. It is likely that the photoluminescent properties of
1a and 2 are characteristic of the dppn unit. The
observation that the emission of 2 in dichloromethane is at higher
energy than 1a is in agreement with an assignment of emissive
states derived from the σ→π*(naphthalene) transition, since
introduction of the methyl group would enhance the hyperconjugation effect,
leading to an increase in the σ→π*(naphthalene) transition
energy.
CR)2],9d in which an assignment of the emission
origin as derived from states of σ→π*(naphthalene) transition
is suggested. It is likely that the photoluminescent properties of
1a and 2 are characteristic of the dppn unit. The
observation that the emission of 2 in dichloromethane is at higher
energy than 1a is in agreement with an assignment of emissive
states derived from the σ→π*(naphthalene) transition, since
introduction of the methyl group would enhance the hyperconjugation effect,
leading to an increase in the σ→π*(naphthalene) transition
energy.
| Complex | Medium (T/K) | λabs/nm (ε/dm3 mol−1 cm−1) | λem/nm (τo/μs) |
|---|---|---|---|
| [Au(dppn)2]PF61a | Solid (298) | 640 (3.2) | |
| Solid (77) | 653 | ||
| CH2Cl2 (298) | 294 (35 870), 398(sh) | 693 (0.7) | |
| (5000) | |||
| [Au(4-Me-dppn)2]PF62 | Solid (298) | 642 (1.3) | |
| Solid (77) | 647 | ||
| CH2Cl2 (298) | 300 (33 720), 400(sh) | 666 (1.5) | |
| (5330) |
Besides exhibiting photoluminescence (PL) behaviour, both 1a and 2 are also found to show electroluminescence (EL) when doped in polycarbonate (PC) as the emissive layer in a single-layered EL device (ITO|Au–PC|Al).† Upon being forward biased with the ITO electrode at positive polarity, the EL devices exhibit intense orange emission. The EL and PL spectra of 2 in thin films are shown in Fig. 2. The close resemblance of the EL and PL spectra suggests that the EL of these four-coordinate Au(I) complexes probably involves the same excited state as PL, i.e. triplet state of σ→π*(naphthalene) transition. The current density–voltage and EL intensity–voltage characteristics of the two single-layered EL devices, (ITO|1a–PC|Al) and (ITO|2–PC|Al), are shown in Fig. 3. The EL intensity exhibits an approximately linear relationship with current density and has a turn-on voltage of ca. 7 V. The EL intensities of the devices of 1a and 2 at 13 V were 82 and 73 cd m−2 with an estimated external quantum efficiency of 0.02 and 0.01% respectively.
 | ||
| Fig. 2 EL (———) and PL (······) spectra 2 in thin films. | ||
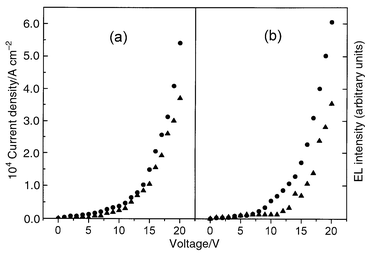 | ||
| Fig. 3 Current density–voltage (•) and EL intensity–voltage (▲) curves for EL devices of ITO|1a–PC|Al (a) and ITO|2–PC|Al (b). | ||
Acknowledgements
V. W.-W. Y. acknowledges financial support from the Research Grants Council and The University of Hong Kong, C.-L. C. the receipt of Croucher Foundation Studentship and Scholarship, K. M.-C. W. the receipt of a research associateship supported by the Vice-Chancellor’s Development Fund of The University of Hong Kong, and E. C.-C. C., S. W.-K. C., S.-C. Y. and P.-K. N. the receipt of a postgraduate studentship from The University of Hong Kong.References
- M. C. Gimeno and A. Laguna, Chem. Rev., 1997, 97, 511 CrossRef CAS; Gold: Progress in Chemistry, Biochemistry and Technology, ed. H. Schmidbaur, John Wiley & Sons, Chichester, 1999and references therein. Search PubMed.
- P. G. Jones, J. Chem. Soc., Chem. Commun., 1980, 1031 RSC; R. C. Elder, E. H. K. Zeiher, M. Onady and R. R. Whittle, J. Chem. Soc., Chem.Commun., 1981, 900 RSC; P. G. Jones, G. M. Sheldrich, J. A. Muir, M. M. Muir and L. B. Pulgar, J. Chem. Soc., Dalton Trans., 1982, 2123 RSC; P. G. Jones, Acta Crystallogr. Sect. C, 1992, 48, 1487 CrossRef; J. M. Forward, Z. Assefa, R. J. Staples and J. P. Fackler Jr., Inorg. Chem., 1996, 35, 16 CrossRef CAS.
- (a) R. Usón, A. Laguna, J. Vicente, J. Garcia, P. G. Jones and G. M. Sheldrich, J. Chem. Soc., Dalton Trans., 1981, 655 RSC; (b) P. A. Bates and J. M. Waters, Inorg. Chim. Acta, 1984, 81, 151 CrossRef CAS; (c) S. J. B. Price, M. A. Mazid and P. J. Sadler, J. Chem. Soc., Dalton Trans., 1984, 969 RSC; (d) A. L. Balch, M. M. Olmstead, P. E. Reedy Jr. and S. P. Rowley, Inorg. Chem., 1988, 27, 4289 CrossRef CAS.
- (a) O. M. N. Dhubhghaill, P. J. Sadler and R. Kuroda, J. Chem. Soc., Dalton Trans., 1990, 2913 RSC; (b) S. J. B. Price, P. S. Jarrett and P. J. Sadler, Inorg. Chem., 1987, 26, 3074 CrossRef.
- (a) R. F. Ziolo, S. Lipton and Z. Dori, J. Chem. Soc., Chem. Commun., 1970, 1124 RSC; (b) C. King, J.-C. Wang, M. N. I. Khan and J. P. Fackler Jr., Inorg. Chem., 1989, 28, 2145 CrossRef CAS; (c) A. Vogler and H. Kunkeley, Chem. Phys. Lett., 1988, 150, 135 CrossRef CAS; (d) V. W.-W. Yam, T.-F. Lai and C.-M. Che, J. Chem. Soc., Dalton Trans., 1990, 3747 RSC; (e) Z. Asefa, B. G. McBurnett, R. J. Staples, J. P. Fackler Jr., B. Assmann, K. Angermaier and H. Schmidbaur, Inorg. Chem., 1995, 34, 75 CrossRef; (f) M. A. Mansour, W. B. Connick, R. J. Lachicotte, H. J. Gysling and R. Eisenberg, J. Am. Chem. Soc., 1998, 120, 1329 CrossRef CAS; (g) W.-F. Fu, K.-C. Chan, V. M. Miskowski and C.-M. Che, Angew. Chem., Int. Ed., 1999, 38, 2783 CrossRef CAS; (h) L. H. Gade, Angew. Chem., Int. Ed. Engl., 1997, 36, 1171 CrossRef CAS; (i) V. W.-W. Yam and K. K.-W. Lo, Chem. Soc. Rev., 1999, 28, 323 and references therein. Search PubMed.
- (a) C.-M. Che, H.-K. Yip, V. W.-W. Yam, P.-Y Cheung, T.-F. Lai, S.-J. Shieh and S.-M. Peng, J. Chem. Soc., Dalton Trans., 1992, 427 RSC; (b) T. M. McCleskey and H. B. Gray, Inorg. Chem., 1992, 31, 1733 CrossRef CAS; (c) C. King, M. N. I. Khan, R. J. Staples and J. P. Fackler Jr., Inorg. Chem., 1992, 31, 3236 CrossRef CAS; (d) S.-J. Shieh , S.-M. Peng and C.-M. Che, J. Chem. Soc., Dalton Trans., 1993, 195 RSC; (e) M. N. I. Khan, R. J. Staples, C. King, J. P. Fackler Jr. and R. E. P. Winpenny, Inorg. Chem., 1993, 32, 5800 CrossRef CAS; (f) V. W.-W. Yam and W.-K. Lee, J. Chem. Soc., Dalton Trans., 1993, 2097 RSC; (g) J. M. Forward, Z. Assefa and J. P. Fackler Jr., J. Am. Chem. Soc., 1995, 117, 9103 CrossRef CAS.
- J. H. Burroughes, D. D. C. Bradley, A. R. Brown, R. N. Marks, K. Mackay, R. H. Friend, P. L. Burn and A. B. Holmes, Nature, 1990, 347, 539 CrossRef CAS; A. Kraft, A. C. Grimsdale and A. B. Holmes, Angew. Chem., Int. Ed., 1998, 37, 402 and references therein. Search PubMed.
- (a) C. W. Tang and S. A. VanSlyke, Appl. Phys. Lett., 1987, 51, 913 CrossRef CAS; (b) C. Adachi, S. Tokito, T. Tsutsui and S. Saito, Jpn. J. Appl. Phys., 1988, 28, L269; (c) J.-K. Lee, D. S. Yoo, E. S. Handy and M. F. Rubner, Appl. Phys. Lett., 1996, 69, 1686 CrossRef CAS; (d) M. A. Baldo, D. F. O’Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Nature, 1998, 395, 151 CrossRef CAS; (e) X. Gong, P.-K. Ng and W.-K. Chan, Adv. Mater., 1998, 10, 1337 CrossRef CAS; (f) Y. Ma, X. Zhou, J. Shen, H.-Y. Chao and C.-M. Che, Appl. Phys. Lett., 1999, 74, 1361 CrossRef CAS; (g) Y. Ma, C. M. Che, H.-Y. Chao, X. Zhou, W.-H. Chan and J. Shen, Adv. Mater., 1999, 11, 852 CrossRef CAS.
- (a) T. E. Müller, S. W.-K. , Choi, D. M. P. Mingos, D. Murphy, D. J. Williams and V. W.-W. Yam, J. Organomet. Chem., 1994, 484, 209 CrossRef; (b) V. W.-W. Yam, S. W.-K. Choi and K. K. Cheung, J. Chem. Soc., Dalton Trans., 1996, 3411 RSC; (c) V. W.-W. Yam, S. W.-K. Choi and K. K. Cheung, Organometallics, 1996, 15, 1734 CrossRef CAS; (d) V. W.-W. Yam and S. W.-K. Choi, J. Chem. Soc., Dalton Trans., 1996, 4227 RSC.
- V. W.-W. Yam, S. W.-K. Choi and K. K. Cheung, Chem. Commun., 1996, 1173 RSC.
- P. D. Harvey and H. B. Gray, J. Am. Chem. Soc., 1988, 110, 2145 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: characterisation data and crystal structure refinement and data for 1b, experimental details for EL measurements. See http://www.rsc.org/suppdata/cc/a9/a908521a/ |
| ‡ Crystal data for [Au(dppn)2]Cl: M = 1225.47, monoclinic, space group P21/n (no. 14), a = 16.981(4), b = 13.527(4), c = 26.399(4) Å, β = 98.75(2)°, V = 5993(2) Å3, Z = 4, Dc = 1.358 g cm−3, μ(Mo-Kα) = 26.53 cm−1, F(000) = 2464, T = 298 K. Convergence for 666 variable parameters by least-squares refinement of F with w = 4Fo2/ς2(F o2), where ς2(Fo2) = [ς2(I) + (0.036Fo2)2] for 4753 reflections with I > 3ς(I) was reached at R = 0.060 and wR = 0.088 with a goodness-of-fit of 2.79. CCDC 182/1487. See http://www.rsc.org/suppdata/cc/a9/a908521a/ for crystallographic files in .cif format. |
| This journal is © The Royal Society of Chemistry 2000 |
