First triphenylene based non-symmetric donor–acceptor triple mesogen possessing disc-like and rod-like characteristics
Swen
Mahlstedt
a,
Dietmar
Janietz
*a,
Andreas
Stracke
b and
Joachim H.
Wendorff
b
aUniversität Potsdam, Fachbereich Chemie und, Institut für Dünnschichttechnologie, Kantstr. 55, D-14513, Teltow, Germany.. E-mail: janietz@rz.uni-potsdam.de
bUniversität Marburg, Institut für Physikalische Chemie und, Wissenschaftliches Zentrum für
Materialwissenschaften, Hans-Meerweinstr., D-35032, Marburg, Germany
First published on 24th December 1999
Abstract
The synthesis and thermal properties of a novel donor–acceptor mesogen which is composed of a disc-like triphenyl ene donor group linked to a flat trinitrofluorenone acceptor via a rod-shaped azobenzene moiety are presented.
Thermotropic liquid crystals may be composed of molecules possessing rod-shaped as well as disc-like anisometric geometry. The molecular shape is known to determine the type of mesophase that is formed. Rod-like mesogens predominantly form smectic phases whereas molecules possessing on average a flat rigid core surrounded by a certain number of long flexible side chains preferably organize into columnar mesophases. A disadvantage for certain applications is that disc-like and rod-like mesogens are not miscible and that phase separation occurs on a macroscopic scale. Even the chemical linkage of molecular sub-units with different anisometric shapes via flexible spacers1–3 does not necessarily lead to mesomorphic properties.
On the other hand, self-organization with formation of liquid crystalline structures can arise from attractive intermolecular interactions such as hydrogen bonding or charge-transfer complex formation between complementary molecules. For example, CT interactions between flat aromatic electron donors such as triphenylene ethers or radial multialkynylbenzene compounds with acceptor molecules may cause the stabilization as well as the induction of columnar mesophases.4–6
The combination of the two principles, molecular geometry and CT interactions, has led to the design of charge-transfer twin molecules with a flat electron donor group separated from an intramolecular acceptor function by a flexible alkyl spacer.7–9 It could be shown that the chemical linkage of the donor with an acceptor molecular sub-unit gives rise to liquid crystalline structures that cannot be achieved by simple mixing of the components.
The concept is that the covalent linking of a disc-like donor to a calamitic moiety, with structure formation tendencies promoted by an additional intramolecular acceptor function, will avoid destruction of mesomorphic order. New aspects for the control of supramolecular structures may arise from this combination of three structural elements, differing in shape and functionality, within one molecule. Recently we reported the first compounds aimed to realize this concept. These are charge-transfer molecules with a calamitic group placed between an enlarged pentaalkynylbenzene donor and a flat acceptor moiety. The novel compounds were found to display a nematic-columnar like phase with the individual columns separated by rod-like groups.10
We present here the non-symmetric trimer 4 which is the first disc-rod triple mesogen composed of a disc-like alkoxy-substituted triphenylene donor group and a nitrofluorenone based acceptor unit, the two complementary parts being linked chemically via alkyl spacers to the termini of a rigid rod-shaped azobenzene moiety.
As shown in Scheme 1, the reaction sequence leading towards the donor–acceptor triple molecule 4 starts out from the alkoxytriphenylene 1 carrying one terminal bromine substituent. Compound 1 was converted into the non-symmetric dimer 3, which combines a disc-like triphenylene donor and a rod-like sub-unit via an ether bridge, by reaction with the azobenzene derivative 2.† Subsequent esterification of the remaining free aliphatic hydroxy group in the intermediate 3 with 3-(2,4,7-trinitrofluoren-9-ylideneaminooxy)propionic acid7 in the presence of N,N′-dicyclohexylcarbodiimide and catalytic amounts of 4-dimethylaminopyridine yielded the triphenylene based trimer 4.‡
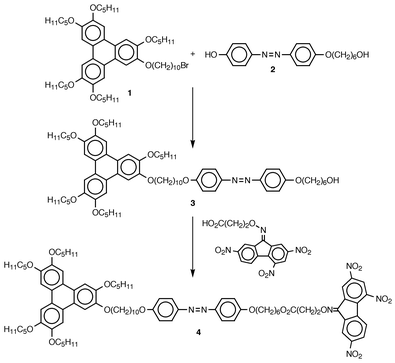 | ||
| Scheme 1 Scheme 1 | ||
Differential scanning calorimetry (DSC) gives evidence that the triphenylene donor–acceptor molecule 4 exhibits a glass transition at 19.4 °C followed by a phase transition into the isotropic phase at 56.3 °C (ΔHi = 1.5 kJ mol−1). Enantiotropic mesomorphic behaviour is confirmed by polarizing microscopy. The optical textures, however, are small-sized and non-specific. Considering the fact that there is no indication of liquid crystalline phase formation for the disc-rod dimer 3 it follows that, as found for charge-transfer triple mesogens based on a flat pentaalkynylbenzene donor moiety,10 the mesophase of the triphenylene CT-triple 4 predominantly originates from donor–acceptor interactions due to the additional chemical linkage of the acceptor sub-unit. Further work is in progress to characterize charge-transfer complex formation of compound 4 spectroscopically.
The wide angle X-ray diffractogram of the triphenylene trimer 4 is presented in Fig. 1. It displays a distinct reflection in the small angle region corresponding to a distance of 1.56 nm. The value agrees approximately with the diameter of the triphenylene donor group. The amorphous halo which appears at larger scattering angles points to the liquid-like ordering of the flexible alkoxy chains. The halo is superimposed on an intense reflection which is characteristic for a periodical packing of flat anisometric cores in a column. The intracolumnar distances amount to 0.34 nm. This value is typical for columnar mesophases of hexaalkoxytriphenylenes doped with electron acceptors such as 2,4,7-trinitrofluoren-9-one (TNF).4
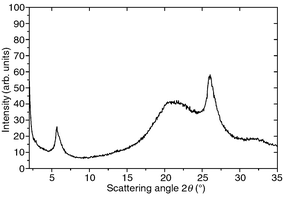 | ||
| Fig. 1 Wide angle X-ray diffractogram of the triphenylene disc-rod triple mesogen 4. | ||
Certainly, the individual columns are formed through an intercalated stacking of the flat donor and acceptor groups of different molecules 4. Furthermore, it is reasonable to suggest that the alkyl spacers connecting the three rigid molecular sub-units preferably adopt more or less stretched conformations. This gives an arrangement of the calamitic moieties with their long axis predominantly oriented orthogonal rather than parallel to the column axis. The distance of 1.56 nm, corresponding to the small angle reflection, would be quite reasonable for the mean distance between adjacent columns perpendicular to the main axis of the molecules. We further have to take into account the unusually high viscosity that is observed by polarizing microscopy within the mesophase range of the triphenylene triple molecule 4, which is typical for liquid crystalline polymers rather than for low molar mass mesogens. Therefore, we assume that as a result of the covalent linkage a one-dimensional quasi-chain incorporating the rod-shaped azobenzene units is realized which connects the alternating stacks of the donor and acceptor fragments of the molecules. A two-dimensional structure model arising from these considerations is presented schematically in Fig. 2.
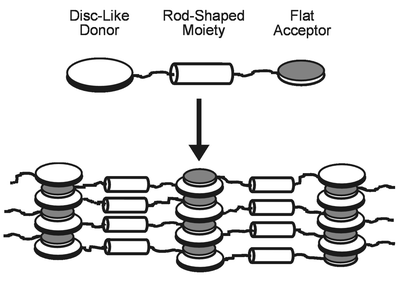 | ||
| Fig. 2 Two-dimensional structure model for the novel mesophase of the donor–acceptor molecule 4 combining features of calamitic liquid crystalline phases with those of columnar mesomorphic structures. For sake of clarity the peripheral alkoxy substituents of the triphenylene donor are not shown. The appearance of only one small angle reflection along with the relatively small clearing transitional enthalpy seem to indicate a strongly distorted structure. Therefore, the model has to be considered as an ‘ideal’ structure at a local molecular level. | ||
The absence of mixed reflections in the small angle region indicates that the columns are not arranged on a two-dimensional lattice, rather that a layer structure exists along one direction. Further investigations are in progress to clarify this point.
In summary, the CT-triple compound 4 is the first representative of a novel family of non-conventional thermotropic liquid crystals arising from the chemical linkage of a disc-like triphenylene donor, a rod-shaped moiety and an acceptor sub-unit. The concept of combining the three different structural elements in a defined manner within one molecule along with charge-transfer interactions introduced by the intramolecular acceptor function provides a powerful tool towards novel thermotropic mesophases, bridging the gap between smectic layer structures and columnar liquid crystalline phases.12 Interesting properties such as optical biaxiality may be expected for such intermediate phases. Further work is currently in progress to modify molecular parameters such as the number of methylene groups in the spacer segments separating the three rigid moieties of the triphenylene based donor-acceptor triple mesogens.
Acknowledgements
The financial support of the Deutsche Forschungsgemeinschaft and of the Land Brandenburg is gratefully acknowledged.References
- W. Kreuder, H. Ringsdorf, O. Hermann-Schönherr and J. H. Wendorff, Angew. Chem., 1987, 99, 1300 CAS.
- O. Karthaus, H. Ringsdorf, M. Ebert and J. H. Wendorff, Makromol. Chem., 1992, 193, 507 Search PubMed.
- I. D. Fletcher and G. R. Luckhurst, Liq. Cryst., 1995, 18, 175 CrossRef CAS.
- H. Bengs, M. Ebert, O. Karthaus, B. Kohne, K. Praefcke, H. Ringsdorf, J. H. Wendorff and R. Wüstefeld, Adv. Mater., 1990, 2, 141 CrossRef CAS.
- M. Ebert, G. Frick, C. Baehr, J. H. Wendorff, R. Wüstefeld and H. Ringsdorf, Liq. Cryst., 1992, 11, 293 CAS.
- K. Praefcke and J. D. Holbrey, J. Inclusion Phenom. Mol. Recognit. Chem., 1996, 24, 19 CAS.
- M. Möller, V. Tsukruk, J. H. Wendorff, H. Bengs and H. Ringsdorf, Liq. Cryst., 1992, 12, 17.
- D. Janietz, Chem. Commun., 1996, 713 RSC.
- D. Goldmann, S. Mahlstedt, D. Janietz, P. Busch, C. Schmidt, A. Stracke and J. H. Wendorff, Liq. Cryst., 1998, 24, 881 CrossRef CAS.
- S. Mahlstedt, D. Janietz, C. Schmidt, A. Stracke and J. H. Wendorff, Liq. Cryst., 1999, 26, 1359 CrossRef CAS.
- N. Boden, R. J. Bushby and Z. B. Lu, Liq. Cryst., 1998, 25, 47 and references cited therein. Search PubMed.
- With respect to lamellar columnar phases, see: K. Ohta, H. Muroki, A. Takagi, K. Hatada, H. Ema, I. Yamamoto and K. Matsuzaki, Mol. Cryst. Liq. Cryst., 1986, 140, 131; Search PubMed; H. Sakashita, A. Nishitani, Y. Sumiya, H. Terauchi, K. Ohta and I. Yamamoto, Mol. Cryst. Liq. Cryst., 1988, 163, 211 Search PubMed.
Footnotes |
| † The 2-(6-bromodecyloxy)-3,6,7,10,11-pentapentyloxytriphenylene 1 used as the starting material was available via ferric chloride mediated regioselective coupling of 3,3′,4,4′-tetrapentyloxybiphenyl with 2-(6-bromodecyloxy)-1-pentyloxybenzene (ref. 11). The azobenzene compound 2 was prepared by alkylation of 4-acetylaminophenol with 6-chlorohexan-1-ol followed by acidic N-acetate cleavage, diazotisation of the resulting aniline and coupling with phenol. |
‡ All new
compounds 1–4 had spectroscopic data (IR,
1H NMR, 13C NMR) consistent with the assigned
structure. Selected data for 4: δH(300
MHz, CDCl3) 8.90 (s, 1H, TNF), 8.56 (m, 2H, TNF), 8.25 (d, 1H,
TNF, J 8.75) 8.03 (d, 1H, TNF, J 8.76), 7.70 (d, 4H,
phenyl, J 8.71), 7.62 (s, 6H, phenyl), 6.89 (m, 4H, phenyl), 4.80
(t, 2H, CH2-O-N, J 6.16), 4.15–4.24 (m, 12H,
CH2-O-phenyl and 2H, CH2-O-CO), 3.95–4.02 (m,
4H, CH2-O-phenyl), 2.95 (t, 2H, CH2-COO, J
6.15), 1.25–2.05 (m, 54H, CH2), 0.99 (t, 15H,
CH3, J 7.05); δC(300 MHz,
CDCl3): 170.50 (1C, COO), 161.10 (2C, phenyl C-O), 160.89 (1C,
TNF C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) N), 148.65 (6C, phenyl C-O), 148.42 (1C, TNF
C-NO2), 146.64 (1C, TNF C-NO2), 146.56 (2C, phenyl
C-N), 143.65 (1C, TNF C-NO2), 138.85 (1C, TNF), 133.67 (1C,
TNF), 131.25 (1C, TNF), 128.34 (1C, TNF CH), 128.78 (1C, TNF CH), 125.79
(1C, TNF), 124.13 (4C, phenyl CH), 122.72 (6C, phenyl and 1C, TNF CH),
120.52 (1C, TNF CH), 119.48 (1C, TNF CH), 114.48 (2C, phenyl CH), 114.43
(2C, phenyl CH), 106.26 (6C, phenyl CH), 72.75 (1C,
CH2-O–N), 69.29 (6C, CH2-O-phenyl), 68.20 (1C,
CH2-O-phenyl), 67.88 (1C, CH2-O-phenyl), 64.94 (1C,
CH2-O-CO), 34.26 (1C, CH2-CO-O), 29.52–22.57
(27C, CH2), 14.10 (5C, CH3);
ν(KBr)/cm−1 1760 (COO), 1530 (NO2), 1340
(NO2). N), 148.65 (6C, phenyl C-O), 148.42 (1C, TNF
C-NO2), 146.64 (1C, TNF C-NO2), 146.56 (2C, phenyl
C-N), 143.65 (1C, TNF C-NO2), 138.85 (1C, TNF), 133.67 (1C,
TNF), 131.25 (1C, TNF), 128.34 (1C, TNF CH), 128.78 (1C, TNF CH), 125.79
(1C, TNF), 124.13 (4C, phenyl CH), 122.72 (6C, phenyl and 1C, TNF CH),
120.52 (1C, TNF CH), 119.48 (1C, TNF CH), 114.48 (2C, phenyl CH), 114.43
(2C, phenyl CH), 106.26 (6C, phenyl CH), 72.75 (1C,
CH2-O–N), 69.29 (6C, CH2-O-phenyl), 68.20 (1C,
CH2-O-phenyl), 67.88 (1C, CH2-O-phenyl), 64.94 (1C,
CH2-O-CO), 34.26 (1C, CH2-CO-O), 29.52–22.57
(27C, CH2), 14.10 (5C, CH3);
ν(KBr)/cm−1 1760 (COO), 1530 (NO2), 1340
(NO2). |
| This journal is © The Royal Society of Chemistry 2000 |
