Synthesis and characterization of cross-conjugated polyenynes
Yuming
Zhao
,
Robert
McDonald
and
Rik R.
Tykwinski
*
Department of Chemistry, University of Alberta, Edmonton, Alberta, Canada T6G 2G2. E-mail: rik.tykwinski@ualberta.ca
First published on 6th January 2000
Abstract
Extended, cross-conjugated polyenynes are reported as well as a description of their electronic (UV–VIS) characteristics and an X-ray crystallographic analysis of hexayne 9 that shows solid-state molecular alignment suitable for topochemical polymerization.
The sequential combination of sp and/or sp2 hybridized carbons affords a series of wire-like π-conjugated carbon chains typified by the structures of polyacetylene (1),1 polydiacetylene (PDA, 2)2,3 and polytriacetylene (PTA, 3)4 in a progression that ultimately concludes with the one-dimensional carbon allotrope, carbyne (4).5–7
The most recent addition to this mélange of enyne oligomers is iso-polydiacetylene (iso-PDA, 5),8 the cross-conjugated isomer of 2.9 Studies of iso-PDAs have shown that π-electron communication is present along the cross-conjugated framework, albeit to a much lesser extent than for PDAs. To provide a better understanding of the physical and electronic characteristics of cross-conjugated enynes, we have extended the skeleton of 5 by inserting additional alkyne groups. Herein, we report the first synthesis of these cross-conjugated polyenynes and a preliminary description of their electronic, X-ray crystallographic and solid-state chemical characteristics.
The enyne oligomers were synthesized as outlined in Scheme 1. Vinyl triflate 610 was coupled with triisopropylsilylacetylene in THF at ambient temperature. Work-up and flash column chromatography, gave the triyne 7 in 93% yield.† Protodesilylation of 7 gave terminal alkyne 8, which was then oxidatively homocoupled11 to hexayne 9 in 96% yield as a stable, light yellow solid.
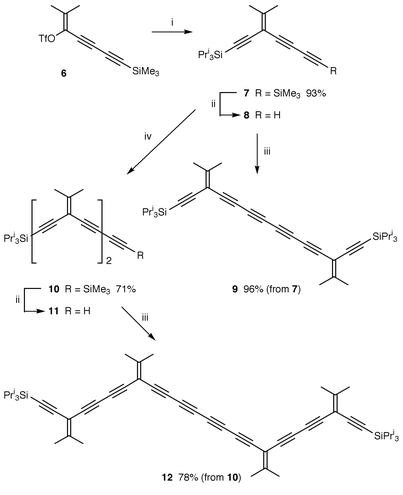 | ||
| Scheme 1 Reagents and conditions: i, triisopropylsilylacetylene, PdCl2(PPh3)2, CuI, Pri2NH, THF, room temp; ii, K2CO3, wet MeOH–THF (1∶1), room temp; iii, CuI, TMEDA, O2, CH2Cl2 room temp; iv, 6, PdCl2(PPh3)2, CuI, Pri2NH, THF, room temp. | ||
Triyne 8 was also elaborated to pentayne 10 in 71% yield via reaction with vinyl triflate 6, as described for the formation of 7. Desilylation of 10 gave the terminal alkyne 11, which afforded a good yield of decayne 12. Notably, the 13C NMR of 12 shows individual resonances for all 10 acetylenic carbons, as well as four unique signals for the alkylidene methyl carbons.‡
Yellow single crystals of hexayne 9 were grown by diffusion of MeOH into a CH2Cl2 solution at –20 °C, and the structure was determined by X-ray crystallographic analysis (Fig. 1).§ The π-framework of 9 is virtually planar, with a maximum deviation of 0.130(17) Å from the least-squares plane for the 16-carbon conjugated skeleton.¶ All six triple bonds are essentially the same length, suggesting little bond length alteration as a result of π-conjugation and contrasting results for PDA oligomers of similar size.12 The eight tetrayne carbons are virtually linear, showing only a gradual curvature with bond angles deviating less than 3° from 180°.
 | ||
| Fig. 1 (a) ORTEP drawing (20% probability level) of 9, (b) crystal packing of 9 as viewed along the b-axis, and (c) crystal packing parameters for 9. | ||
Analysis of the crystal packing of 9 down the b-axis shows the molecules are aligned in a parallel fashion (Fig. 1). The intermolecular proximity of the tetraynes in the crystal suggests the potential for topochemical polymerization as is well established for suitably aligned butadiynes and hexatriynes.2,13,14a The solid-state polymerization of octatetraynes has also been previously described, albeit in much less detail.14
The parameters describing the intermolecular relationship of molecules 9 are depicted in Fig.1(c).2,13a,14a Polymerization of 9via 1,2- or 1,4-addition, as described for tetrayne systems,14 is unlikely considering that R1,2 (6.7 Å) and R1,4 (4.7 Å) are both considerably more than the ideal distance of 4 Å between reacting carbon atoms. The angle phi between the tetrayne rod and the stacking axis at ca. 28° is significantly less than the optimum value of 45° determined for 1,4-addition in di-, tri- and tetra-ynes.2,13a,14a Thus, two alternative addition patterns might occur for 9, namely 1,6-addition (R1,6 = 3.6 Å) and 1,8-addition (R1,8 = 4.2 Å). The stacking angle phi is nearly identical to the value of 27° that denotes highest 1,6-reactivity in triacetylenes, and the stacking distance between monomer units at 7.7 Å is quite near the desired value of 7.5 Å.13a In view of these values, 1,6-addition is the expected mode of reaction for 9.
Differential scanning calorimetry showed that crystals of 9 have a well-defined melting point at 103–104 °C that is followed by decomposition at 145 °C. Attempts to thermally effect polymerization of single crystalline 9 were conducted at 90 °C. Over the course of 8 h, the crystals darken slightly to yield an opaque solid. A loss of crystal integrity for the resultant material was confirmed by the absence of any X-ray diffraction pattern. To date, the product(s) of this thermal reaction has not been identified.
Photochemical polymerization of a single crystal of 9 was attempted by monochromatic irradiation at 280 nm.15,16 After approximately 2 h, a darkening of the crystal to yellow–orange was observed. Irradiation was continued for another 4 h with little additional darkening. X-Ray analysis of the crystal afforded a diffraction pattern and cell parameters identical to that of pure 9, indicating little change in the solid beyond that which may have occurred at the outer surface of the crystal.
The electronic absorption spectra of 9, 12 and 1,8-bis(4-tert-butylphenyl)-octa-1,3,5,7-tetrayne 13, are shown in Fig. 2. The lower energy region is dominated by the absorption pattern of the tetrayne moiety and is remarkably similar to that of 13.17 Each compound displays three absorptions at ca. 405, 374 and 348 nm. Whereas the UV spectra of elongated iso-PDAs show evidence of π-electron communication via cross-conjugation,8 this effect is absent in 9 and 12, as the three lowest energy absorptions of 12 are each red-shifted by only 1 nm versus those of 9.
 | ||
| Fig. 2 UV–VIS spectra in CHCl3 comparing polyynes 9, 12 and 13. | ||
In conclusion, cross-conjugated polyenynes 7–12 can be synthesized in high yields as relatively stable solids. The UV–VIS spectra of 9 and 12 show a minimal contribution from cross conjugation. The solid state organization of 9 is suitable for topochemical polymerization, although initial attempts to effect thermal and photochemical polymerization have been unsuccessful. Further studies on 9 and 12 are currently underway.
Acknowledgements
This work was supported by a Gen-Science Endowment from the University of Alberta and by NSERC. We thank Professor M. M. Haley for providing a sample of 13.References
-
Conjugated Polymers and Related Materials: The Interconnection of
Chemical and Electronic Structure, ed. W. R. Salaneck, I.
Lundström and B. Rånby, OUP,
Oxford, 1993. Search PubMed
.
- V. Enkelmann, Adv. Polym. Sci., 1984, 63, 91 Search PubMed
.
- G. Wegner, Makromol. Chem., Suppl., 1984, 6, 347 Search PubMed
.
- R. E. Martin, T. Mäder and F. Diederich, Angew. Chem., Int. Ed., 1999, 38, 817 CrossRef CAS
.
-
(a) P. P. K. Smith and P. R. Buseck, Science, 1982, 216, 984 CAS
; (b) R. Hoffmann, Tetrahedron, 1966, 22, 521 CrossRef CAS
.
-
(a) R. J. Lagow, J. J. Kampa, H.-C. Wei, S. L. Battle, J. W. Gegne, D. A. Laude, C. J. Harper, R. Bau, R. C. Stevens, J. F. Haw and E. Munson, Science, 1995, 267, 362 CAS
; (b) R. Eastmond, T. R. Johnson and D. R. M. Walton, Tetrahedron, 1972, 28, 4601 CrossRef CAS
.
-
(a)
T. B. Peters,
J. C. Bohling,
A. M. Arif and
J. A. Gladyz,
Organometallics, 1999, 18,
3261; Search PubMed
; (b) R. Dembinski, T. Lis, S. Szafert, C. L. Mayne, T. Bartik and J. A. Gladysz, J. Organomet. Chem., 1999, 578, 229 CrossRef CAS
; (c) B. Bartik, R. Dembinski, T. Bartik, A. M. Arif and J. A. Gladysz, New J. Chem., 1997, 21, 739 Search PubMed
; (d) J. T. Lin, J. J. Wu, C.-S. Li, Y. S. Wen and K.-J. Lin, Organometallics, 1996, 15, 5028 CrossRef CAS
; (e) M. Altmann, V. Enkelmann and U. H. F. Bunz, Chem. Ber., 1996, 129, 269 CrossRef CAS
.
- Y. Zhao and R. R. Tykwinski, J. Am. Chem. Soc., 1999, 121, 458 CrossRef CAS
.
-
For cross-conjugated PTAs, see: A. M. Boldi, J. Anthony, V. Gramlich, C. B. Knobler, C. Boudon, J.-P. Gisselbrecht, M. Gross and F. Diederich, Helv. Chim. Acta,
1995, 78,
779. Search PubMed
.
- P. J. Stang and M. Ladika, Synthesis, 1981, 29 CrossRef CAS
.
- A. S. Hay, J. Org. Chem., 1962, 27, 3320 CrossRef CAS
.
- R. Giesa and R. C. Schulz, Polym. Int., 1994, 33, 43 CrossRef CAS
.
-
(a)
V. Enkelmann,
Chem. Mater., 1994, 6,
1337; Search PubMed
; (b) J. Kiji, J. Kaiser, G. Wegner and R. C. Schulz, Polymer, 1973, 14, 433 CrossRef CAS
; (c) S. Okada, H. Matsuda, H. Nakanishi, M. Kato and M. Otsuka, Thin Solid Films, 1989, 179, 423 CrossRef CAS
.
-
(a) R. H. Baughman and K. C. Yee, J. Polym. Sci., Macromol. Rev., 1978, 13, 219 Search PubMed
; (b) S. Okada, H. Matsuda, A. Masaki, H. Nakanishi and K. Hayamizu, Proc. SPIE-Int. Soc. Opt. Eng., 1991, 1560, 25 Search PubMed
; (c) K. Hayamizu, S. Okada, S. Tsuzuki, H. Matsuda, A. Masaki and H. Nakanishi, Bull. Chem. Soc. Jpn., 1994, 67, 342 CAS
; (d) A. Sarkar, S. Okada, K. Komatsu, H. Nakanishi and H. Matsuda, Macromolecules, 1998, 31, 5624 CrossRef CAS
.
-
A. E. Keating and
M. A. Garcia-Garibay,
in Organic and Inorganic Photochemistry, ed. V. Ramamurthy
and K. S. Schanze, Marcel Dekker, New
York, 1998. Search PubMed
.
- H. Bässler, Adv. Polym. Sci., 1984, 63, 1 Search PubMed
.
- M. M. Haley, M. L. Bell, S. C. Brand, D. B. Kimball, J. J. Pak and W. B. Wan, Tetrahedron Lett., 1997, 38, 7483 CrossRef CAS
.
Footnotes |
| † The purity and structure of all new compounds were confirmed by 1H and 13C NMR, IR, UV, MS and either EA or HRMS. |
| ‡ Selected data for 9: yellow solid, mp 103–104 °C; δH(300 MHz, CDCl3) 2.07 (s, 6H), 2.05 (s, 6H), 1.07 (s, 42H); δC(75 MHz, CDCl3) 162.3, 101.2, 100.9, 94.9, 76.3, 75.0, 67.8, 64.1, 23.4, 23.2, 18.7, 11.3. For 12: yellow solid, decomposition begins at ca. 80 °C; δH(300 MHz, CD2Cl2) 2.11 (s, 12H), 2.09 (s, 6H), 2.06 (s, 6H), 1.10 (s, 42H); δC(125 MHz, CD2Cl2) 166.6, 160.8, 102.3, 101.2, 99.7, 94.3, 80.2, 77.5, 77.0, 76.9, 75.4, 74.4, 68.1, 64.2, 23.7, 23.6, 23.3, 23.1, 18.8, 11.7. |
§ Crystal data for 9: C38H54Si2, M = 566.99,
monoclinic space group P21/c (No. 14),
Dc = 1.018 g cm–3, Z = 4, a =
13.5571(8), b = 7.7132(5), c = 35.616(2) Å, β
= 96.5990(10)°, V = 3699.7(4) Å3, μ =
0.118 mm−1 Final R(F) = 0.0472,
wR2(F2) = 0.1366 for 365 variables
and 7039 data with Fo2
![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) − 3ς(Fo2) (4842
observations [Fo2
− 3ς(Fo2) (4842
observations [Fo2
![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) 2ς(Fo2)]). CCDC
182/1485.
2ς(Fo2)]). CCDC
182/1485. |
| ¶ The known X-ray structures of tetraynes and a pentayne have been summarized in refs. 7(b) and 7(c). |
| This journal is © The Royal Society of Chemistry 2000 |

