Porous gold structures through templating by echinoid skeletal plates
Fiona C. Meldruma and Ram Seshadrib
aDepartment of Chemistry, Queen Mary and Westfield College, London University, Mile End Road, London, UK E1 4NS. E-mail: F.C.Meldrum@qmw.ac.uk
bSolid State and Structural Chemistry Unit, Indian Institute of Science, Bangalore, 560 012, India.. E-mail: seshadri@sscu.iisc.ernet.in
First published on UnassignedUnassigned6th January 2000
Abstract
Porous gold structures with nearly regular 15 μm channels have been prepared by a novel route in which the calcium carbonate skeletal plates of echinoids (sea urchins) are used as templates.
Biological materials are characterised by unique structures and morphologies that cannot typically be replicated through laboratory synthesis. Nature uses materials not traditionally recognised for their engineering properties, such as calcium carbonate, to produce structures with superior mechanical properties. This is possible owing to control over morphology, composition and spatial organisation. Profiting from existing materials with unusual forms, we describe experiments using a structured calcium carbonate biomineral to template the production of porous inorganic solids (gold in this case) with pore diameters in the order of 15 μm. Few synthetic methods exist to produce materials that are spatially patterned on this length scale.
There is much interest in the preparation of materials with well defined pore sizes. Microporous solids, characterised by pore sizes of the order of 10 Å, include the zeolites. Mesoporous solids exhibit pore sizes in the range 10–1000 Å and represent a relatively new class of materials. A number of methods have been used to synthesise mesoporous materials based on surfactant templating.1–5 These have been applied to a wide range of inorganic materials, including metals, silicates and metal oxides.1–5 Reports on the synthesis of porous solids with pores in the micrometer size range are considerably more limited. Lamellar aluminophosphates with surface patterns on the sub-micrometer to millimeter size range have been produced using vesicle templates.6 Bicontinuous microemulsions have been used to prepare porous shells of crystalline calcium carbonate with pore sizes ranging from 35 to 225 nm,7 and calcium phosphate materials with microstructures comprising pore diameters of up to several micrometers.8 The method of emulsion templating9 is perhaps most general and has been used to produce macroporous titania, silica and zirconia with pore sizes from 50 nm to several microns.
Here we describe the use of the skeletal plates of echinoids (sea urchins) to template porous structures with pore sizes of the order of 15 μm.† The example material chosen here is gold, but the method is quite general. A continuous coating of gold is deposited over the entire surface area of the skeletal plates. Subsequent dissolution of the plate leaves the gold coating in the form of the original structure. Sea urchin plates were selected owing to their unique morphology. The skeletal plates and the spines of echinoids10 are a low/high magnesium calcite (depending on genetic and geographical control) and each behave as single crystals when examined by diffraction or polarised light. The microstructure, however, is quite remarkable and complex, exhibiting a unique fenestrated structure of interconnected trabeculae and pores that are approximately 15 μm in diameter [Fig. 1(a)]. The porous network and inorganic fractions of the shell occupy approximately equal volumes, and display non-crystallographic curved surfaces. Indeed, the pore diameters and pore size distributions are highly controlled, and some species of urchin are characterised by highly uniform distributions of pores in very regular structures.11
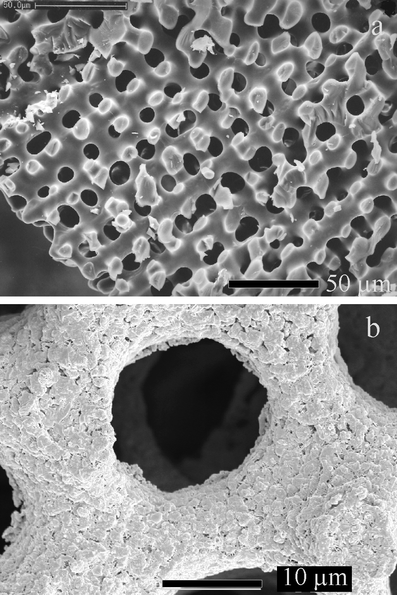 | ||
| Fig. 1 Scanning electron micrographs of (a) a cleaned echinoid skeletal shell and (b) shell coated with gold and annealed. The bars correspond to (a) 50 μm and (b) 10 μm. | ||
A typical SEM image in cross section through a skeletal plate is presented in Fig. 1(a), showing the bicontinuous, sponge-like structure of the mineral with a length scale in the order of 15 μm. The mineral surface is very smooth even when viewed at higher magnification (not shown). Fig. 1(b) shows a similar skeletal plate after gold coating and annealing. The coating of gold particles on the surface of the mineral is clearly seen as a very marked roughening. Dissolution of the calcium carbonate component of a gold-coated echinoid plate produced the gold structures shown in Fig. 2(a) and (b). These images demonstrate that the echinoid plate acts as a template to produce porous gold with a structure mirroring that of the original plate. The gold particles produce a coating on the calcite, rather than filling the entire pore structure, so a double-sided surface is produced. This is clearly shown in Fig. 2(b), where the gold surface originally in contact with the calcite is smooth (area 1) while the surface directed into the original pore is rough (area 2).
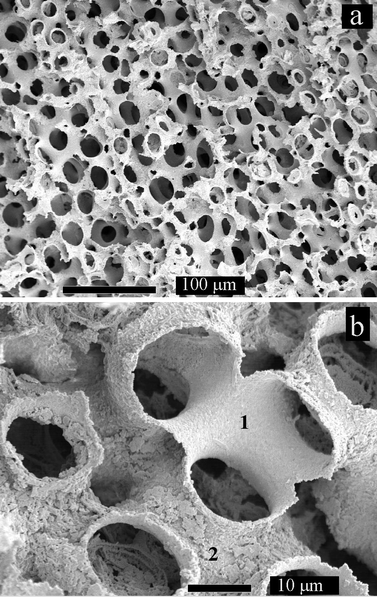 | ||
| Fig. 2 SE micrographs of gold-coated echinoid skeletal shell after in situ dissolution of the calcium carbonate shell. 1 and 2 in (b) mark the smooth inner and rough outer surfaces of the templated gold structure. The bars correspond to (a) 100 μm and (b) 10 μm. | ||
Dissolution of the gold-coated plates in acid solution produced a solid of the same dimensions as the original plate. The solid produced was rather fragile and thus difficult to transfer to sample stubs without some damage to the structure. The best images of the porous gold were thus obtained from samples dissolved in situ on the sample stub. Further work will be directed to producing thicker metal coatings and even filling the entire pore structure to produce a replica of the original bicontinuous structure. This should produce a stronger material.
It is demonstrated that the skeletal plates of echinoids can be used to template the synthesis of porous materials (gold being chosen as an example) with unique structures. The technique is very general and it is envisaged that it can be applied to a wide range of inorganic solids. Indeed, preliminary experiments to produce macroporous TiO2 structures via a sol–gel method suggest that the method is feasible, depending upon refinement of the kinetics of the reaction and control of shrinkage during annealing. Further experiments to deposit a wider range of solids and to control the thickness of the templated material are underway.
The class of new materials presented here may find application as light-weight structural ceramics. Ordered macroporous materials with pore dimensions comparable to optical wavelengths could display unique optical properties. Applications as catalyst supports are also possible, particularly in situations where the traditional materials with smaller pore sizes result in unacceptable pressure drops in the catalysis process.
Acknowledgements
R. S. thanks the RSC for a Journals Grant. We thank the Department of Materials, QMW College, for allowing access to electron microscope facilities.References
- Q. Huo, D. I. Margolese, U. Ciesla, P. Feng, T. E. Gier, P. Sieger, R. Leon, P. M. Petroff, F. Schüth and G. D. Stucky, Nature, 1994, 368, 317 CrossRef CAS.
- K. M. McGrath, D. M. Dabbs, N. Yao, I. A. Aksay and S. M. Gruner, Science, 1997, 277, 552 CrossRef CAS.
- N. K. Raman, M. T. Anderson and C. J. Brinker, Chem. Mater., 1996, 8, 1682 CrossRef CAS.
- Q. Huo, D. I. Margolese and G. D. Stucky, Chem. Mater., 1996, 8, 1147 CrossRef CAS.
- G. S. Attard, M. Edgar and C. G. Göltner, Acta Mater., 1998, 46, 751 CrossRef CAS.
- S. Oliver, A. Kuperman, N. Coombs, A. Lough and G. A. Ozin, Nature, 1995, 378, 47 CrossRef CAS.
- D. Walsh and S. Mann, Nature, 1995, 377, 320 CrossRef CAS.
- D. Walsh, J. D. Hopwood and S. Mann, Science, 1994, 264, 1576 CrossRef CAS.
- A. Imhof and D. J. Pine, Nature, 1997, 389, 948 CrossRef CAS.
- P. Dubois and C.-P. Chen, in Echinoderm Studies, ed. M. Jangoux and J. M. Lawrence, A. A. Balkema, Rotterdam, 1989, vol. 3, p. 109. Search PubMed.
- G. Donnay and D. L. Pawson, Science, 1969, 166, 1147 CrossRef.
- D. M. Swift, S. Sikes and A. P. Wheeler, J. Exp. Zool., 1986, 240, 65 Search PubMed.
Footnote |
| † Specimens of
the echinoid Ircinia oros were collected at Depot Beach, NSW,
Australia, and were cleaned of organic material with hypochlorite solution
using standard procedures.12 Experiments
were carried out with clean skeletal plates that had been abraded to a
thickness of ca. 1 mm, sonicated in water to remove attached
particles and subsequently dried. One end of a dry plate was then placed in
gold paint (Johnson Matthey, Brilliant Gold, PM 002), and the paint was
absorbed into the plate via capillary action, as evidenced from a
colour change in the plate from white to dark brown–purple. The plate
was then removed from the paint and was heated using a hot air gun to burn
off the protective organic matter in the paint. The plate was allowed to
cool slightly before repetition of the dipping/heating cycle. The cycle was
repeated ten times and was then annealed in a furnace at 400 °C for 36
h. The samples were examined using a JEOL 6300F scanning electron microscope (SEM), fitted with a field emission source and operating at 5 kV. Samples were prepared for (SEM) by mounting on aluminium stubs using adhesive carbon tape, either with the original outer surface of the plate exposed or in cross-section after fracture, and all were sputter-coated with a thin layer of gold prior to viewing to enhance conductivity. Pieces of echinoid plates were examined after cleaning [Fig. 1(a)] and subsequent to annealing of the absorbed gold [Fig. 1(b)]. Samples were also studied after dissolution of the calcium carbonate with acid. The annealed gold plates were immersed in solutions of 2% acetic acid or 2 M HCl until all of the original calcite plate has been dissolved, and the remaining gold material was mounted on SEM stubs. Plates were also mounted on SEM stubs and were dissolved in stiu with 2 M HCl [Fig. 2(a) and (b)]. |
| This journal is © The Royal Society of Chemistry 2000 |
