Reusable photographic films based on iron-containing clay minerals
Yea Wenn Liou and Chong Mou Wang*
Department of Chemistry, National Taiwan Normal University, Taipei, 11718, Taiwan, Republic of China.. E-mail: checmw@scc.ntnu.edu.tw
First published on UnassignedUnassigned7th January 2000
Abstract
A non-silver-based photographic film has been developed from 9-methylacridinium-intercalated clay particles (denoted clay|AcH+) and this inexpensive clay film showed potential reusability.
Substantial efforts have recently been directed towards the development of artificial photosynthetic systems,1–5 which, in turn, unite the understanding of the structure of natural photosynthetic systems, the advances in instrumentation, and the development of novel materials for optical and chemical detection.6–8 For the latter aspects, we recently carried out a series of investigations on clay modified electrodes, and found that iron-containing clay minerals proved useful in many applications, such as biosensing and optical recognition.9–12 The iron containing species are likely to be key in mediating the electron reactions on the clay surface.13,14 Besides, we also found that this inexpensive solid, if properly tailored, may be useful for lithography or image-recording.
Photography can be dated back to 1727 as Johann Heinrich Schulze reported that solid AgCl darkens when exposed to light. Thereafter, most color and black-and-white photography was developed on the basis of the photochemistry of AgBr. A representative example [eqn. (1)] is the following coupling reaction between α-naphthanol and N,N-diethyl-2-methyl-1,4-phenylenediamine (DEPD) sensitized by AgBr:15,16
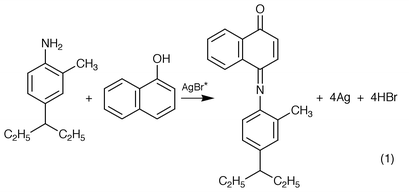 | (1) |
Meanwhile, many other dyes have showed excellent sensitivity to colored objects or to images only visible under IR or UV light. However, for most cases, silver bromide is sacrificed and the film is not recoverable. Here we describe that a potentially reusable photographic film can be developed from inexpensive iron-containing clay minerals.
When the clay|AcH+ modified electrode is simultaneously biased at +0.4 V vs. SCE and illuminated with light of λ > 350 nm (100 W Hg lamp, 1 min), Fig. 1 shows that indophenol blue (λabs ≈ 625 nm) is immediately formed on the surface of the clay film from a solution containing DEPD (λabs,max = 257 nm, pH 10) and α-naphthanol (λabs = 250, 337 nm, pH 10). Here, the clay|AcH+ particles were prepared by reacting clay (Aldrich, montmorillonite K10, 0.5 g) with AcHPF6 (10 mM, 50 mL in CH3CN)13 for two days followed by extensive washing with CH3CN; the loading of AcH+ in clay was estimated to be ca. 10−5 mol g−1. The clay-modified electrode was fabricated on conductive ITO glass (indium-doped, 0.7 mm thick, 20 Ω cm−2; Delta Technol.). Typically, a portion of the clay|AcH+ slurry (30 μL cm−2, 100 mg/100 mL water) was spin-coated onto glass sheets and dried at room temperature. Then, the clay film was reinforced with polystyrene [PS, Mw 13000, Aldrich, 30 μL cm−2, 10 mg mL−1 in ethyl acetate–toluene (1∶1, v/v)]. The mechanism of formation of indophenol blue on the clay surface is proposed in Scheme 1 and control experiments support the proposed mechanism. As is evident in Fig. 2, insignificant photocurrent is detected for electrodes made from the bare clay particles in a pH 4 solution containing 1 mM of DEPD, whereas a greater photocurrent is obtained when the electrode is incorporated with AcH+ (λabs,max = 357 nm; λem = 500 nm). Laser flash photolysis experiments (Fig. 3) show that the lifetime of AcH+*(aq) (dotted lines) is 36 ns whereas the decay for AcH+*(clay) (solid lines) occurs in the same time scale as the instrumental response (≪10 ns), indicating that the excited acridinium, AcH+*(clay), can be quickly quenched by iron species, presumably Fe2+, in the clay particle, at FeII sites.13 The fast decay of AcH+* suggests a reductive quenching mechanism [eqn. (2)].
| AcH+*(clay) + Fe2+(clay) → AcH.(clay) + Fe3+(clay) | (2) |
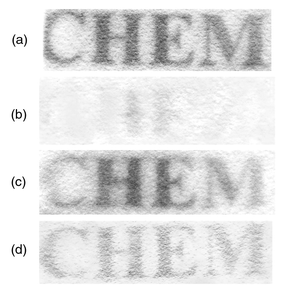 | ||
| Fig. 1 (a) Photoimage formed on the clay|AcH+ modified electrode in a solution containing 2 mM of DEPD and 3 mM of α-naphthanol (pH 4) under illumination (λ > 350 nm) and at E = 0.4 V vs. SCE for 1 min; (b) image erasing, after the image obtained in (a) was dipped in a dilute sulfuric acid for 1 s; (c) image recovering, the electrode in (b) was re-exposed to light in solution (a); (d) the image obtained after 20 consecutive exposure and erase cycles. | ||
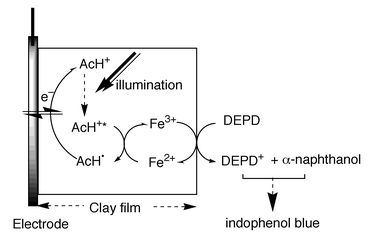 | ||
| Scheme 1 Proposed mechanisms for the formation of indophenol blue on the illuminated clay|AcH+ modified electrode. | ||
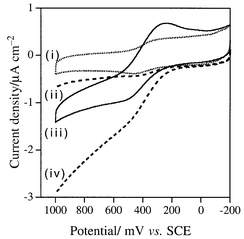 | ||
| Fig. 2 J–V curves for DEPD (1 mM, pH 4): (i) with the electrode prepared with the bare clay particles, in the dark; (ii) with the clay|AcH+ electrode, in the dark; (iii) with the electrode as in (a), under illumination; (iv) with the clay|AcH+ electrode as in (b), under illumination. Scan rate: 50 mV s−1. | ||
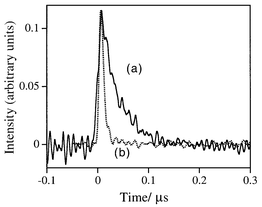 | ||
| Fig. 3 Time-resolved laser spectroscopy (pH 4, acetic acid–acetate buffer, I ≈ 0.1 M, λem = 500 nm) for free AcH+ (a) and clay|AcH+ (b). | ||
The photoquality in terms of the contrast between the blue regions and the background is a function of the electrode potential, pH and the concentration ratio of the reactants. For instance, a high pH environment greatly favored the formation of indophenol blue. However, for a solution pH of >6, the formation of indophenol blue became too rapid to be controlled. As a consequence, a lower pH environment (pH 4), although less favorable for the formation of indophenol blue on the clay electrode, was, in turn, less interfering. The electrode potential is another key factor determining the image quality. Control experiments showed that the formation of indophenol blue tended to a limiting rate as the electrode potential approached 0.4 V vs. SCE (i.e. the formal potential of FeIII/II(clay))10 according to the absorbance monitored at 625 nm (the peak absorption of indophenol blue). This effect is attributed to the regeneration of FeIII(clay) from FeII(clay)[eqn. (3)]
| FeII(clay) → FeIII(clay) + e− | (3) |
| FeIII(clay) + DEPD → FeII(clay) + DEPD+ | (4) |
This filmed electrode appeared to be reusable since the image could be erased after dipping in dilute (1 mM) sulfuric acid [Fig. 1(b)] and once the electrode was reexposed a clear image could be restored [Fig. 1 (c)]. Long-term tests [Fig. 1 (d)], in addition, showed that although the clay|AcH+ electrode gradually loses its sensitivity (tentatively ascribed to leaching of AcH+), it could be reused more than twenty times. In summary, such very cheap iron-containing clay minerals after proper tailoring, may be useful materials for image-recording.
We thank the National Science Council, Republic of China and Chinese Petroleum Company for financial support (grant number: NSC 88-2113-M-003-016; NSC 88-CPC-M-003-002).
References
- G. L. Closs and J. R. Miller, Science, 1988, 240, 440 CrossRef CAS.
- M. R. Wasielewski, D. G. Johnson, W. A. Svec, K. M. Kersey, D. C. Cragg and D. W. Minsek, in Photochemical Energy Conversion, ed., J. R. Norris, Jr. and D. Mieisel, Elsevier, New York, 1989, pp. 1135–1147. Search PubMed.
- D. Gust and T. A. Moore, Science, 1989, 244, 35 CrossRef CAS.
- M. R. Wasielewski, M. P. Niemczyk, W. A. Svec and E. B. Pewitt, J. Am. Chem. Soc., 1985, 107, 5562 CrossRef CAS.
- E. Danielson, C. M. Elliot, J. W. Merkert and T. J. Meyer, J. Am. Chem. Soc., 1987, 109, 2519 CrossRef CAS.
- M. A. Fox, Adv. Photochem., 1986, 13, 237 Search PubMed.
- Chemical Sensors, ed., T. E. Edmonds, Chapman & Hall, New York, 1988. Search PubMed.
- U. Schaller, E. Bakker, U. E. Spichiger and E. Pretsch, Anal. Chem., 1994, 66, 391 CrossRef CAS.
- S.-C. Shyu and C. M. Wang, J. Electrochem. Soc., 1997, 144, 3419 CAS.
- S.-C. Shyu and C. M. Wang, J. Electroanal. Chem., 1997, 440, 27 CrossRef CAS.
- S.-C. Shyu and C. M. Wang, J. Electrochem. Soc., 1998, 145, 154 CAS.
- C. F. Cheng and C. M. Wang, J. Electroanal. Chem., 1999, 466, 82 CrossRef.
- Y. W. Teng, I.-J. Chang and C. M. Wang, J. Phys. Chem. B, 1997, 101, 10386 CrossRef CAS.
- C. S. Ouyang and C. M. Wang, J. Electrochem. Soc., 1998, 145, 2654 CAS.
- R. D. Theys and G. Sosnovsky, Chem. Rev., 1997, 97, 83 CrossRef CAS.
- Braving the Elements, ed., H. B. Gray, J. D. Simon and W. C. Trogler, University Science Books, Sausalito, 1995, pp. 300–314. Search PubMed.
| This journal is © The Royal Society of Chemistry 2000 |
