New chiral heterogeneous catalysts based on mesoporous silica: asymmetric diethylzinc addition to benzaldehyde
Sung
Jin Bae
a,
Sang-Wook
Kim
b,
Taeghwan
Hyeon
*b and
B.
Moon Kim
*a
aDepartment of Chemistry and, Center for Molecular Catalysis, Seoul National University, Seoul, 151-742, Korea.. E-mail: kimbm@plaza.snu.ac.kr
bSchool of Chemical Engineering, Seoul National University, Seoul, 151-742, Korea.. E-mail: thyeon@plaza.snu.ac.kr
First published on 7th January 2000
Abstract
New heterogeneous catalyst systems have been developed employing a proline-derived ligand immobilized on mesoporous silicas; asymmetric diethylzinc addition to benzaldehyde was carried out using the catalyst systems; the reaction enantioselectivity was found to be largely dependent upon the pore size of the mesoporous silicas, capping of free silanol moieties with trimethylsilyl group and employment of BunLi.
Optically active secondary alcohols are important intermediates for the construction of optically active natural products and biologically active compounds. Among various methods for the construction of optically active secondary alcohols, addition of diorganozinc reagents to aldehydes in the presence of a catalytic amount of a chiral β-aminoalcohol has been extensively studied.1 Extremely high enantioselectivity has been reported in the reactions employing a variety of aminoalcohol ligands. However, in order for these reactions to be successfully exploited in large-scale applications, the chiral ligand has to be readily available or easily recycled. Toward this goal, immobilization of chiral ligands onto heterogeneous supports has been investigated and a number of support materials such as inorganic solids and polymers have been developed.2 Recently, various mesoporous silicas with pore sizes ranging from 2 to 10 nm have been synthesized using self-assemblies of surfactants and block copolymers as templates.3 These mesoporous silicas have been successfully employed as supports for various chiral catalysts.4 Herein, we report on new heterogeneous catalyst systems that utilize proline derivatives anchored on the mesoporous silicas such as MCM-41 and SBA-15.
Although the reactions of diethylzinc with benzaldehyde using ephedrine immobilized onto amorphous silica gel5 and mesoporous silica6 have been investigated, the degree of enantioselection under these heterogeneous catalytic conditions has been less than satisfactory. In connection with the low enantioselectivty, several issues need to be addressed: (1) employment of a more selective ligand, (2) suppressing undesired catalytic activity on the silica surface, (3) changing silica pore sizes, and (4) addition of extra metal reagent. In terms of a more selective ligand system, a prolinol-based ligand developed by Soai et al.7 was selected in our study. Since the free SiOH moieties of the silica surface could catalyze the reaction,6 thus lowering the reaction enantioselectivity, mesoporous silicas containing the chiral ligand having its surface capped with trimethylsilyl groups8 have been prepared. To investigate the effect of the pore size on the enantioselectivity, we have used two different mesoporous silicas, MCM-41 and SBA-15, which have similar hexagonal pore arrays but with different pore dimensions. We have also investigated reaction enantioselectivity when the catalyst system was treated with BunLi before addition of the aldehyde.
The synthetic procedure including preparation of the ligand, immobilization onto silicas and capping with trimethylsilyl moieties is shown in Scheme 1. The new chiral aminoalcohol 3 was synthesized as follows. The amine group of 4-hydroxyproline (Aldrich Chemicals™) was protected using ethyl chloroformate and the acid was converted to a methyl ester to give the carbamate ester 2. Treatment of compound 2 using phenylmagnesium chloride furnished a tertiary alcohol, and the carbamate group was converted to the corresponding methylamine through treatment with lithium aluminium hydride (LAH) yielding compound 3 in an overall yield of 72% from 1. MCM-41 and SBA-15 were synthesized according to the previously reported procedures.3 Chloropropyl linkers were grafted on the walls of mesoporous silicas by treating with (CH3CH2O)3Si–CH2CH2 CH2Cl in refluxing toluene. Reaction of the resulting chloropropyl-derivatized mesoporous silicas (5b and 5c) with the chiral pyrrolidinemethanol 3 yielded silicas 6b and 6c, respectively. In order to cap remaining free SiOH groups on the walls of mesoporous silicas, 5b and 5c were treated in refluxing hexamethyldisiloxane (HMDS) for 12 h. To the best of our knowledge, this is the first anchoring of SBA-15 silica with a chiral ligand and the X-ray diffraction (XRD) patterns of SBA-15 derivatized samples have been taken during various stages of the preparation. The XRD results clearly demonstrated that the mesoporous structure of SBA-15 was preserved during the preparation of the catalyst. Table 1 lists the surface areas, the mean pore dimensions, and the amounts of grafted organic groups for the mesoporous silica based catalysts. The surface area decreased substantially during the chloropropyl-grafting step, especially for SBA-15. Nonetheless, the pore dimensions of the SBA-15 based systems did not change significantly during these synthetic steps. For comparison, the chiral ligand 3 was attached to amorphous silica to provide both non-TMS capped catalyst (6a) and TMS-capped catalyst (8a) using the same reaction conditions applied to the mesoporous silicas.
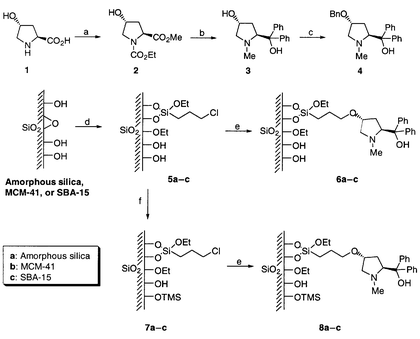 | ||
| Scheme 1 The synthetic routes used in the preparation of various catalysts. Reagents and conditions: a: (i) ethyl chloroformate, NaHCO3, H2O, room temp., 16 h; (ii) SOCl2, MeOH, room temp., 12 h; b: (i) PhMgCl, THF, 0 °C, 5 h; (ii) LiAlH4, THF, reflux, 3 h; c: NaH, BnBr, THF, room temp., 16 h; d: Chloropropyltriethoxysilane, toluene, reflux, 12 h; e: 3, xylene, reflux, 12 h; f: HMDS, reflux, 12 h | ||
| MCM-41 based catalysts | SBA-15 based catalysts | ||||||
|---|---|---|---|---|---|---|---|
| Catalyst | Surface area/ m2 g−1 | Mean pore diameter/nm | Grafted amount/ mmol g−1 | Catalyst | Surface area/ m2 g−1 | Mean pore diameter/nm | Grafted amount/ mmol g−1 |
| a N2 adsorption and desorption isotherms were collected at STP on a Micrometrics ASAP2010 gas adsorption analyzer after the materials were degassed at 250 °C at 30 μTorr for 5 h. The surface areas were calculated by the BET method and the pore size distributions were calculated from the adsorption branch of the nitrogen isotherm by the BJH method. | |||||||
| Silica | 919 | 3.1 | Silica | 800 | 8.9 | ||
| 5b | 750 | 3.0 | 0.55 | 5c | 472 | 8.5 | 1.38 |
| 6b | 655 | 2.4 | 0.40 | 6c | 320 | 8.4 | 0.36 |
| 8b | 648 | 2.3 | 0.59 | 8c | 312 | 8.4 | 0.37 |
We have investigated asymmetric addition of diethylzinc to benzaldehyde using these silica-based catalysts and the results are outlined in Table 2. For each silica catalyst, two different sets of reactions were run with 6 mol% of catalyst and either 3 equiv. of diethylzinc to benzaldehyde (method A) or 7.2 mol% of BunLi followed by 3 equiv. of diethylzinc (method B). To evaluate our catalyst systems against the parent homogeneous catalyst, compound 4 was prepared from 3 through etherification of C(4)–OH with benzyl bromide. Reactions employing the homogeneous catalyst 4 provided products with 90 and 93% ee, respectively, via methods A and B (entries 1 and 2, Table 2). However, when the chiral catalysts anchored on amorphous silica were tested using method A, 6a and 8a gave only 16 and 37% ee, respectively (Table 2 entries 4 and 5). When the reactions were carried out using method B, slight improvements in the enantioselectivities were observed (entries 6 and 7). A noticeably higher enantioselectivity was observed in the reactions employing MCM-41-based catalyst (entries 8–11) and the best results were obtained with the catalysts based upon SBA-15 (entries 12–15). It is of particular note that catalysts based upon SBA-15 consistently gave higher enantioselectivity than that of MCM-41. In both MCM-41 and SBA-15 systems, TMS-capping as well as the employment of BunLi improved the enantioselectivity significantly. Product of the highest ee (75%) was obtained for TMS-capped SBA-15 based catalyst utilized after treatment with BunLi (entry 15). To test the catalytic activity of the silica without a chiral ligand, TMS-capped SBA-15 (7c) was used under otherwise the same reaction conditions, and a 15% yield of the product was observed. It appears that, even with TMS-capping, there remains some residual activity of the silica, resulting in a reduction of the enantioselectivity.
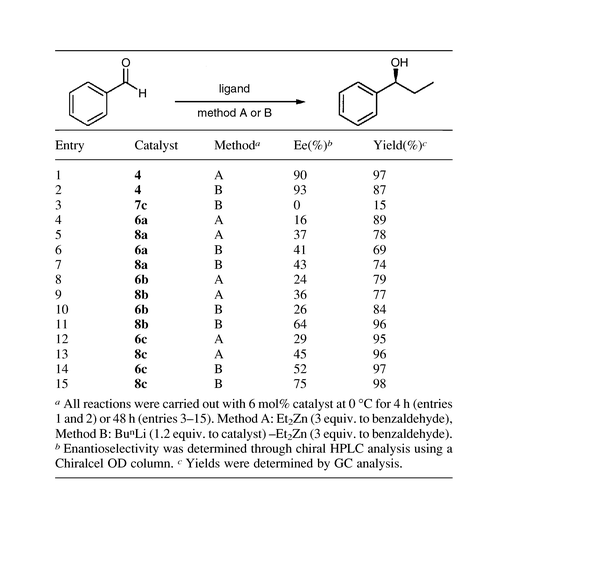
In summary, new mesoporous silica-based catalysts incorporating a chiral pyrrolidinemethanol derivative have been prepared and the asymmetric diethylzinc addition to benzaldehyde examined. Among various catalytic systems, the best result (75% ee) was obtained with the TMS-capped SBA-15 based catalyst treated with BunLi.
Acknowledgements
T. H. would like to thank KISTEP (Critical Technology 21) and Korea Institute of Science and Technology (KIST) for the financial support and BASF-Korea for supplying P123 triblock copolymer. B. M. K. acknowledges generous financial support from the Korea Science and Engineering Foundation (97-0501-0201-3) and the KOSEF through the Center for Molecular Catalysis at Seoul National University.References
- R. Noyori and M. Kitamura, Angew. Chem., Int. Ed. Engl., 1991, 30, 49 CrossRef; K. Soai and S. Niwa, Chem. Rev., 1992, 92, 833 CrossRef CAS.
- Heterogeneous Catalysis and Fine Chemicals IV, ed. H. Blaser, A. Baiker and R. Prins, Elsevier, Amsterdam, 1997; Search PubMed; J. H. Clark and D. J. Macquarrie, Chem. Commun., 1998, 853; Search PubMed; Handbook of Heterogeneous Catalysis, ed. G. Ertl, H. Knözinger and J. Weitkamp, Wiley-VCH, Weinheim, 1997, vol. 1. Search PubMed.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef CAS; D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS; J. Y. Ying, C. P. Mehnert and M. S. Wong, Angew. Chem., Int. Ed., 1999, 38, 57 CrossRef.
- X. G. Zhou, X. Q. Yu, J. S. Huang, S. G. Li, L. S. Li and C. M. Che, Chem. Commun.., 1999, 1789 RSC; B. F. G. Johnson, S. A. Raynor, D. S. Shephard, T. Mashmeyer, J. M. Thomas, G. Sankar, S. Bromley, R. Oldroyd, L. Gladden and M. D. Mantle, Chem. Commun., 1999, 1167 RSC; G.-J. Kim and J.-H. Shin, Tetrahedron Lett., 1999, 40, 6827 CrossRef CAS.
- K. Soai, M. Watanabe and A. Yamamoto, J. Org. Chem., 1990, 55, 4832 CrossRef CAS.
- M. Laspéras, N. Bellocq, D. Brunel and P. Moreau, Tetrahedron: Asymmetry, 1998, 9, 3053 CrossRef CAS.
- K. Soai, A. Ookawa, T. Kaba and K. Ogawa, J. Am. Chem. Soc., 1987, 109, 7111 CrossRef CAS.
- T. Tatsumi, K. A. Koyano, Y. Tanaka and S. Nakata, J. Phys. Chem. B., 1997, 101, 9436 CrossRef.
| This journal is © The Royal Society of Chemistry 2000 |
