Sampling of kidneys from cattle and pigs for cadmium analysis†
Ing-Marie Olsson* and Agneta Oskarsson
Department of Pharmacology and Toxicology, Swedish University of Agricultural Sciences, Box 573, Uppsala, SE-751 23, Sweden
First published on 18th December 2000
Abstract
Cadmium accumulates in proximal tubule cells causing a gradient of cadmium through the kidney, which is important to consider when sampling kidney tissue for cadmium analysis. In this study different sampling techniques of cattle and pig kidneys have been tested. Cadmium was determined by dry ashing–FAAS (detection limit 6.0 μg l−1, BCR (Community Bureau of Reference) No. 186 3.1 ± 0.17 mg kg−1 (mean ± s), laboratory quality sample (LQS) 495 ± 17 μg kg−1) and microwave digestion–graphite furnace AAS (detection limit 0.24 μg l−1, BCR No.186 2.7 ± 0.16 mg kg−1, LQS 444 ± 14 μg kg−1) in homogenates, slices, and in cortex, intermediate and medulla zones of bovine and porcine kidneys. The bovine kidney lobulus cortex, intermediate zone, and medulla contained 70, 28 and 2% of the total cadmium content, and the relative weights of the zones were 53, 35 and 12%, respectively. The cadmium concentration in bovine cortex, intermediate zone and medulla was 1.37 ± 7, 0.79 ± 0.06 and 0.10 ± 0.06 times the calculated homogenate concentration. Pig renal cortex, intermediate zone and medulla, contained 73, 26 and 0.5% respectively of the total cadmium content, and the relative weights were 63, 36 and 2.4%, respectively. The cadmium concentration in porcine cortex, intermediate zone and medulla was 1.14 ± 0.05, 0.78 ± 0.09 and 0.23 ± 0.11 times the calculated homogenate concentration. Freezing of pig kidney caused a slight redistribution of cadmium from cortex to medulla. The results show that sampling technique is of greater importance for the determination of cadmium in bovine kidney than in pig kidney. A well described method for sampling of kidney is necessary to make it possible to compare results. To detect small differences in renal Cd levels between groups, as, e.g., in the case of biological monitoring of Cd exposure, sampling of the outer cortex is suggested as an optimal method.
Introduction
Cadmium (Cd) is a toxic metal that accumulates in kidney tissue. The kidney parenchyma consists of cortex and medulla. There are gross- and micro-anatomical differences between kidneys of different species.1 Kidneys have a gradual change of dominant cell type from cortex to medulla. Number of nephrons as well as proportions of the different nephron classes vary for different species. Due to the anatomy of the kidney and the fact that Cd mainly accumulates in the proximal tubule cells2–4 a gradient of Cd is seen through the kidney. This gradient has to be considered when sampling kidney tissue for Cd determination.Information on the distribution of Cd within human, pig and cattle kidney is scarce.5–9 Details in the literature about the sampling procedure of kidney for Cd analyses vary, all the way from just stating that kidneys have been sampled, over homogenisation of parts of or whole kidney, to extraction of cortex.10–17 To be able to compare data from different studies not only quality control of the analytical method is needed but also a standardised and reproducible sampling method of representative samples. Another analytical problem is contamination of samples during sample preparation. Homogenisation may, as well as, e.g., scissors and knives, introduce contaminants. The aim of this study was to develop a practical sampling technique of kidneys from cattle and pigs for cadmium determination with the intention of biomonitoring, and to increase the knowledge of cadmium distribution within bovine and porcine kidneys.
Experimental
Sampling of bovine kidney
Kidneys were collected from nine cows, slaughtered and immediately bled at the abattoir in Uppsala, Sweden. The kidneys were freed from visible fat, large vessels and connective tissue. One kidney from four individuals was used for homogenates. The lobuli of the other kidney were separated and frozen separately in plastic bags until further analysis. The following sampling procedures were compared: One kidney each from four cows (No. 2–5) were cut into 10, eight, six and four slices, respectively [Fig. S1 available as electronic supplementary information (ESI)†]. Each slice was homogenised in a Waring blender mixer with a threefold knife-head of stainless steel. The homogenates were frozen at −20 °C in acid washed plastic containers until analysis. Triplicates of homogenates (4.7–9.9 g) were analysed for cadmium by flame atomic absorption spectrometry after dry ashing (DA-FAAS).From one cow (No. 1) five lobuli and from four cows (No. 2–5) four lobuli were sectioned from cortex to hilus into four pieces (S1–S4) (Fig. 1b). The sections [2.4–17.2 g (minimum–maximum weight)] were analysed for cadmium by DA-FAAS.
 | ||
| Fig. 1 (a) Cattle kidney with marked lobuli showing the approximate position of lobuli sampled for cadmium analysis of (b) sections (S1–S4) and (c) cortex, intermediate and medulla zones by DA-FAAS. Marked part of cortex shows the piece extracted for analysis by MW-GFAAS. | ||
From eight animals (No. 2–9), four lobuli, at approximately the same positions (Fig. 1a) within the kidneys, were taken from the freezer, semi-thawed and divided into three zones (Fig. 1c) and weighed; cortex (3.2–19.3 g), intermediate (2.3–14.8 g) and medulla (0.6–5.4 g). From four of the animals (No. 2–5) all the tissue of the lobuli were analysed. Some of these lobuli (n = 7) were too large for the zones to be analysed in one piece. These lobuli were subdivided into 2–4 parts before being split into zones. The results were used to evaluate intra-lobular differences of cadmium concentration. The zones from the remaining four animals (No. 6–9) were prepared from a 5 mm thick slice through the centre of the lobulus. The samples were weighed and analysed for cadmium by DA-FAAS. Samples of medulla that had Cd levels below the limit of detection by FAAS were analysed by graphite furnace AAS (GFAAS).
Comparison of DA-FAAS and microwave-GFAAS
Kidneys were collected from nine cows at the abattoir in Luleå, Sweden, and stored frozen at −20 °C until analysis. Duplicate samples of cortex (0.5 g) from two 5 mm thick slices taken at the centre of a lobulus (Fig. 1c), were analysed for cadmium by GFAAS after microwave digestion (MW-GFAAS). The remainder of the cortex was analysed (1.7–7.0 g) by DA-FAAS.Sampling of pig kidney
Kidneys were collected from nine pigs, slaughtered and immediately bled at the abattoir in Uppsala, Sweden. The kidneys came from one pig slaughtered at approximately 40 kg live weight (No. 10), five pigs at approximately 107 kg (growing/finishing pigs, No. 11–15), and from three sows (No. 16–18). The kidneys were freed from visible fat, large vessels and connective tissue. The following sampling procedures were compared: One kidney from each of the 40 kg pig, two finishing pigs (No. 11–12) and two sows (No. 16–17) was homogenised as described above for the bovine kidney homogenates. Two kidneys were homogenised whole (No. 10 and No. 12) and three homogenised in halves (No. 11, 16 and 17). Triplicates of homogenates (4.6–8.9 g) were analysed by DA-FAAS.One kidney from each of six pigs (No. 10–12 and 16–18) was frozen and stored at −20 °C. The kidneys were semi-thawed and cut into approximately 5 mm thick slices (Fig. 2a) (for individual No. 18 only half the kidney was sliced). Every second slice was sampled by division into two halves, and every second by division into zones (cortex, intermediate, and medulla) (Fig. 2b and c). Cadmium determination was performed by DA-FAAS. Dry-ashed samples of medulla that had Cd levels below the limit of detection by FAAS were analysed by GFAAS.
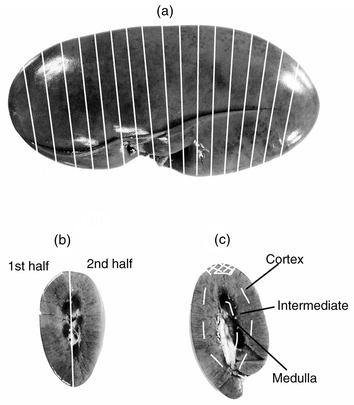 | ||
| Fig. 2 (a) Pig kidney sampled for cadmium (Cd) analysis by flame atomic absorption spectrometry after dry-ashing (DA-FAAS). Lines showing approximate position for slicing. (b) Slice division along the line for analysis of cadmium in corresponding halves of slices. (c) Slice sampled for analysis of cortex, intermediate and medulla zones. Marked part of cortex indicates the piece extracted for analysis by the MW-GFAAS method. | ||
Fresh vs. frozen samples and DA-FAAS compared to MW-GFAAS
From three growing/finishing pigs (No. 13–15) one kidney was analysed as fresh, while the other kidney was analysed after freezing. The kidneys were cut into 5 mm thick slices. From the outer cortex 0.5 g were taken for analysis by MW-GFAAS, the remaining part of every second slice was cut into cortex, intermediate and medulla zones and analysed for cadmium by DA-FAAS (Fig. 2c).Sample preparation and analytical methods
All utensils coming into contact with the samples were soaked in 1.5 mol l−1 nitric acid (pro analysi (p.a.), Merck, Kebo-lab, Spånga, Sweden) for approximately 12 h and then rinsed three times in deionized water and three times in deionized and distilled water. The sterile stainless steel surgical blades (Paragon, 24, No. 4 Fitment, BS 2982, Maersk Medical Limited, Sheffield, Great Britain) used to excise the samples from the kidneys were checked for Cd release by extraction in 0.1 mol l−1 nitric acid (supra pure, Merck) overnight. A 0.9 g per 100 ml NaCl solution was used to check the Waring blender mixer for cadmium release. The saline was mixed for 2 min and then left in the mixer overnight. All the extraction solutions were analysed for Cd and found to be below the limit of detection by GFAAS.Dry ashing–flame atomic absorption spectrometry (DA-FAAS)
Drying and ashing was performed in a Lenton programmable furnace (Market Harborough, Leicestershire, UK). Samples (0.076–19.6 g) were weighed to the nearest 0.001 g, into high shape porcelain crucibles and dried at 103 °C for approximately 48 h (Table S1 available as ESI)† after which the dried samples were weighed to determine the water content. The samples were dry-ashed for 24 h (Table S2 available as ESI)† after which the crucibles were taken out and allowed to cool to room temperature. The ashes were wetted with 3 ml deionized and distilled water and then ashed again for 48 h. This was repeated once to completely combust the organic material. The ashes were weighed after cooling to room temperature in a desiccator and 5 ml 6 mol l−1 hydrochloric acid (supra pure, Merck) was added. The crucibles with the acid–ash mixture were left overnight covered by glass plates. The following day the acid was evaporated on a boiling water-bath. The residues were dissolved in 10 ml 0.1 mol l−1 nitric acid (supra pure) overnight, after which the content was transferred to weighed 50 ml polypropylene sample tubes. The crucibles were rinsed two times with 5 ml 0.1 mol l−1 nitric acid (supra pure), which was added to the sample tube. The sample solutions were weighed. Determination of Cd was performed on a flame atomic absorption spectrophotometer with deuterium lamp background correction (Perkin Elmer, FAAS 4100, Bodenseewerk Perkin-Elmer GmbH, Überlingen, Germany) (Table S3 available as ESI)†. Linear calibration was performed with Cd standards 20, 100 and 500 μg l−1, in 0.1 mol l−1 nitric acid (supra pure), prepared from a Titrisol Cd standard (Merck).MW-GFAAS
Kidney samples (0.5 g) were weighed into acid-washed Teflon digest vessels and 5 ml of concentrated nitric acid (p.a.) was added. The vessels were mounted in the microwave labstation (MLS 1200 H MEGA, Milestone, AB Ninolab, Milestone S.r.l., Sorisole, Italy). One sample was monitored for temperature control during the digestion programme (Table S4 available as ESI)†. The digested samples were transferred to 50 ml polypropylene sample tubes and the Teflon vessels and lids were rinsed three times with 3 ml 0.1 mol l−11 nitric acid (supra pure). The rinse solutions were added to the sample tubes. The kidney sample solutions were then diluted to 50 ml with 0.1 mol l−1 nitric acid (supra pure) and weighed. Determination of Cd was performed by GFAAS with Zeeman background correction (Perkin Elmer, GFAAS 4100ZL) (Table S3 and S5 available as ESI)†. Linear calibration was used with 0.5, 1.0 and 2.0 μg l−1 Cd standards, in 3 mol l−1 nitric acid (p.a.), prepared from a Titrisol Cd standard (Merck).Quality control and limit of detection
Duplicate samples of reagent blanks, certified pig kidney (BCR No. 186, Community Bureau of Reference, Brussels, Promochem AB, Ulricehamn, Sweden) and homogenised bovine kidney [‘laboratory quality sample’ (LQS)], used for internal quality control, were analysed with every sample series. The certified value of BCR No. 186 was 2.71 ± 0.15 mg kg−1 (mean ± 95% confidence interval). Our results averaged 3.1 ± 0.17 mg kg−1 (mean ± s; n = 48) for analysis by DA-FAAS, 2.5 ± 0.18 (n = 21) for analysis by dry-ashing GFAAS (DA-GFAAS) and 2.7 ± 0.16 (n = 21) for analysis by MW-GFAAS. The average Cd concentration for the LQS analysed by DA-FAAS was 495 ± 17 μg kg−1 (n = 48), the precision for the LQS expressed as the repeatability relative standard deviation (RSDr)18 was 2.2%. For LQS analysed by MW-GFAAS the results were 444 ± 14 μg kg−1 (n = 20), with a RSDr of 2.6%.The limit of detection was 6.0 μg l−1 for DA-FAAS [3 standard deviations (s) of 65 reagent blanks (RB)] and 0.32 μg l−1 for DA-GFAAS (3s of 32 RB), 0.24 μg l−1 for MW-GFAAS (3s of 20 RB). The precision expressed as the RSDr was 7.1% for all the kidney analysis. Our laboratory has regularly participated in the proficiency-testing programme of trace elements in food, organised by the Swedish National Food Administration, with a mean Z-score of −0.7 (n = 4).
Calculation of cadmium concentration
For the calculation of the Cd concentration, the exact volume of the sample solution had to be determined. The density of a subset of the dry ashed and the microwave digested samples from reference material, cow and pig kidney, was determined by weighing a definite volume of the sample solution. The weighing procedure was repeated ten times for each sample solution and the mean weight was used to calculate the density of the samples.The total calculated cadmium concentration in cow kidney lobuli and pig kidney slices was calculated as: calculated Cd conc.lobulus/slice = (C1m1 + … + Cnmn)/( m1 + … + mn); with (C1m1 + … + Cnmn) = total cadmium content in lobulus/slice and (m1 + … + mn) = sum of weights of different parts of lobulus/slice.
Statistics
The Statview® 5.0 software (1998, SAS® Institute Inc., Cary, NC, USA) was used. Results were tested for normality by Kolomogorov–Smirnoff and the homogeneity of variances tested by Bartlett’s test. Depending on the outcome of these tests further analysis was performed by unpaired or paired t-test, and ANOVA, or Mann–Whitney U test and Kruskal–Wallis. Post-hoc testing was performed with Games–Howell. The level of significance was set to P ⩽ 0.05.Results and discussion
Comparison of DA-FAAS and MW-GFAAS
The nine bovine kidney and 24 pig kidney cortex samples showed no significant difference in a paired t-test between DA-FAAS {182 ± 125 (91–715) μg Cd kg−1 [mean ± s (minimum and maximum)]} and MW-GFAAS [181 ± 122 (80–699)]. The correlation coefficient for the two methods was 0.996, regression line CdMW-GFAAS = 3.608 + 0.971 × CdDA-FAAS, r2 = 0.992 [sample range 80–715 μg Cd kg−1 wet weight (WW)]. The Cd concentration in the reference material in this set of analyses was 3.00 ± 0.17 mg Cd kg−1 (n = 12) with DA-FAAS and 2.65 ± 0.20 (n = 16) with MW-GFAAS.Bovine kidney
The mean values (μg Cd kg−1 WW) for each individual and sampling method is shown in Table 1.| Individuals | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Part of kidney sampled | No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | No. 7 | No. 8 | No. 9 | Mean |
| a Number of slices homogenised and analysed.b Number of lobuli analysed.c Number of slices, from central location in lobuli, analysed. | ||||||||||
| Homogenate | — | 672 ± 51 | 706 ± 47 | 398 ± 23 | 525 ± 14 | — | — | — | — | |
| (10)a | (8)a | (6)a | (4)a | |||||||
| S1 | 379 ± 15 | 945 ± 111 | 948 ± 26 | 546 ± 24 | 702 ± 60 | — | — | — | — | |
| [% of calculated total | (5)b | (4)b | (4)b | (4)b | (4)b | (21) | ||||
| lobuli cadmium conc.] | [136 ± 6%] | [135 ± 14%] | [137 ± 6%] | [131 ± 4%] | [130 ± 11%] | [134 ± 8%] | ||||
| S2 | 260 ± 22 | 673 ± 54 | 698 ± 54 | 410 ± 22 | 523 ± 31 | — | — | — | — | |
| [% of calculated total | (5)b | (4)b | (4)b | (4)b | (4)b | (21) | ||||
| lobuli cadmium conc.] | [94 ± 10%] | [96 ± 7%] | [101 ± 7%] | [98 ± 1%] | [97 ± 2%] | [97 ± 6%] | ||||
| S3 | 230 ± 31 | 577 ± 17 | 543 ± 64 | 327 ± 39 | 444 ± 47 | — | — | — | — | |
| [% of calculated total | (5)b | (4)b | (4)b | (4)b | (4)b | (21) | ||||
| lobuli cadmium conc.] | [83 ± 8%] | [82 ± 3%] | [79 ± 7%] | [78 ± 7%] | [83 ± 8%] | [81 ± 6%] | ||||
| S4 | 253 ± 42 | 588 ± 133 | 559 ± 81 | 372 ± 23 | 505 ± 62 | — | — | — | — | |
| [% of calculated total | (5)b | (4)b | (4)b | (4)b | (4)b | (21) | ||||
| lobuli cadmium conc.] | [91 ± 12%] | [84 ± 20%] | [81 ± 11%] | [89 ± 7%] | [94 ± 10%] | [88 ± 12%] | ||||
| Cortex | — | 936 ± 29 | 980 ± 41 | 527 ± 16 | 697 ± 33 | 252 ± 6 | 292 ± 37 | 510 ± 6 | 489 ± 3 | |
| [% of calculated total | (4)b | (4)b | (4)b | (4)b | (4)c | (4)c | (4)c | (4)c | (32) | |
| lobuli cadmium conc.] | [134 ± 3%] | [136 ± 10%] | [124 ± 3%] | [134 ± 6%] | [142 ± 3%] | [142 ± 6%] | [144 ± 2%] | [138 ± 3%] | [137 ± 7%] | |
| Intermediate | — | 525 ± 32 | 628 ± 56 | 340 ± 32 | 436 ± 26 | 133 ± 12 | 162 ± 11 | 268 ± 28 | 285 ± 16 | |
| [% of calculated total | (4)b | (4)b | (4)b | (4)b | (4)c | (4)c | (4)c | (4)c | (32) | |
| lobuli cadmium conc.] | [75 ± 4%] | [86 ± 5%] | [80 ± 7%] | [82 ± 4%] | [75 ± 5%] | [79 ± 3%] | [75 ± 8%] | [80 ± 4%] | [79 ± 6%] | |
| Medulla | — | 72.4 ± 14 | 123 ± 26 | 82.8 ± 26 | 98.2 ± 33 | 9.0 ± 2 | 12.0 ± 6 | 16.0 ± 4 | 25.3 ± 10 | |
| [% of calculated total | (4)b | (4)b | (4)b | (4)b | (4)c | (4)c | (4)c | (4)c | (32) | |
| lobuli cadmium conc.] | [10 ± 2%] | [15 ± 2%] | [16 ± 4%] | [18 ± 5%] | [5 ± 1%] | [6 ± 3%] | [5 ± 1%] | [7 ± 3%] | [10 ± 6%] | |
| Calculated value for | 695 | 727 | 424 | 529 | 179 | 209 | 356 | 356 | ||
| homogenate based on | ||||||||||
| analysis of zones | ||||||||||
The Cd levels ranged from 37 to 780 μg Cd kg−1 and were within the span of Cd in bovine kidney homogenates11,17,19–21 and cortex14,15,21,22 earlier reported. However, only in the studies by Koh et al.,14 Lücker et al.,21 and Lopéz Alonso et al.20 are full descriptions of the sampling method given.
The s of the Cd concentration in homogenates of individual slices from the bovine kidneys (Table 1, No. 2–5) was highest in the kidney cut into 10 slices and lowest in the kidney cut into four slices. The anatomical structure of bovine kidneys can explain differences in cadmium concentration of homogenates from the same individual if the piece homogenised is not large enough. Cadmium concentrations for homogenates of half kidneys were calculated in these four animals (No. 2: 655 vs. 670 μg kg−1; No. 3: 687 vs. 716 μg kg−1; No. 4: 392 vs. 395 μg kg−1; No. 5: 515 vs. 535 μg kg−1) showing a difference of borderline significance (P = 0.056, paired t-test) between the halves. The results indicate that whole kidneys ought to be used if homogenates of cattle kidneys are to be analysed.
Analysis of sectioned bovine kidney lobuli (S1–S4, Fig. 1b) showed that S1 had significantly higher cadmium levels than the three other sections (P =0.0013, Kruskal–Wallis). The lowest mean Cd concentration was consistently found in S3, although the levels were not significantly different from S2 and S4. The S2 section concentrations were relatively close to the analysed concentrations of homogenates (No. 2–5), while S1 were relatively close to the analysed Cd concentrations for cortex. The analysed Cd concentration at each level was related to the total lobulus Cd concentration (μg kg−1 WW) as percentage of calculated lobulus cadmium concentration (Table 1). The fairly equal distribution of Cd in the same sections from different individuals indicates that the sampling procedure is relatively reproducible for all sections and that the anatomical structure is similar for different lobuli. The larger standard deviation (s) for S4 might be due to a larger variation in proportion of cortex and medulla at this level of the lobuli.
The zones differ in Cd concentration (P <0.001, Kruskal–Wallis), cortex 247–1020 (min–max) μg Cd kg−1, intermediate 119–626 μg Cd kg−1, and medulla 6.4–127 μg Cd kg−1 (Table 1). The distribution of Cd in the zones was similar for the individual kidneys with approximately 137% in the cortex, 79% in the intermediate and 10% in the medulla of the total lobuli Cd concentration. There were no significant intra-lobular differences in cadmium concentrations within the respective zones (data not shown). A randomly assigned value was used from the subdivided lobuli for further calculations and comparisons. Cadmium concentrations did not differ between different lobuli (n = 4) within an individual (n = 8) (Kruskal–Wallis). This is well in line with the finding of Lücker et al.21 who examined cortex and two parts of the medulla in three bovine kidneys with micro solid sampling and GFAAS.
The water and ash content differs between medulla and the other zones (P <0.0001, Kruskal–Wallis), ranges do however overlap (Table 2). The relative weight of cortex (44%) in the slices is lower than in a whole lobulus (53%) simply for anatomical reasons. A slice from the centre of a lobulus gives a relatively higher content of the intermediate zone than in a whole lobulus. The cortex contained 70 and 62% of the total amount of Cd, while 28 and 37% were found in the intermediate zone and only 2 and 0.4% in the medulla for lobuli and slices, respectively. The lower value for medulla from the slices is probably due to it being easier to prepare separated zones from a slice than from a lobulus.
| Zone of kidney lobuli/slices | ||||
|---|---|---|---|---|
| Parameter | Cortex mean ± s (min–max) | Intermediate mean ± s (min–max) | Medulla mean ± s (min–max) | |
| a Significantly different from cortex and intermediate, P <0.0001, Kruskal–Wallis. | ||||
| Water content (n = 32) | 79.4 ± 1.0 | 79.5 ± 0.8 | 82.9 ± 0.8a | |
| (%) | (77.9–80.9) | (78.1–81.0) | (80.7–84.1) | |
| Ash content (n = 32) | 1.20 ± 0.05 | 1.21 ± 0.05 | 1.31 ± 0.13a | |
| (%) | (1.06–1.29) | (1.10 ± 1.30) | (1.09–1.68) | |
| Relative weight | Lobuli | 53 ± 4.4 | 35 ± 3.3 | 12 ± 2.6 |
| (%) | (n = 16) | (46–63) | (30–42) | (6.7–15) |
| Slices | 44 ± 3.1 | 48 ± 3.4 | 7.5 ± 1.6 | |
| (n = 16) | (38–49) | (44–55) | (3.8–10) | |
| % of total cadmium content | ||||
| Lobuli | 70 ± 3.7 | 28 ± 3.5 | 1.8 ± 0.66 | |
| (n = 16) | (64–77) | (22–34) | (0.73–3.0) | |
| Slices | 62 ± 4.1 | 37 ± 4.1 | 0.44 ± 0.24 | |
| (n = 16) | (53–70) | (29–46) | (0.14–1.1) | |
Pig kidney
The mean values (μg Cd kg−1 WW) for each individual and sampling method is shown in Table 3. The mean concentration of cadmium in homogenates of pig kidneys ranged from 44.8 to 672 μg kg−1. This is within the range reported for Cd in pig kidneys from Slovenia.11 Levels in animals No. 10–12 are within the ranges reported in studies of growing/finishing pigs.8,19 However, a full description of sampling methods is lacking in two of these three reports. The main reasons for the wide concentration range is probably age and Cd levels in feed.23 There were no significant differences between the halves of the homogenates from animals No. 11, 16 and 17 (88.3 vs. 70.5 μg Cd kg−1, 323 vs. 326 μg Cd kg−1, and 663 vs. 680 μg Cd kg−1, paired t-test).| Individuals and animal category at slaughter | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Part of kidney sampled | No. 10 40 kg at slaughter | No. 11 Finishing/ growing pig | No. 12 Finishing/ growing pig | No. 13 Finishing/ growing pig | No. 14 Finishing/ growing pig | No. 15 Finishing/ growing pig | No. 16 Sow | No. 17 Sow | No. 18 Sow | Mean |
| a Number of analyses.b Number of slices analysed. | ||||||||||
| Homogenate | 44.8 ± 5.5 | 79.4 ± 10.5 | 87.3 ± 2.7 | — | — | — | 324 ± 3.3 | 672 ± 16.4 | — | |
| (3)a | (6)a | (3)a | (6)a | (6)a | ||||||
| (Whole) | (Half kidney) | (Whole) | (Half kidney) | (Half kidney) | ||||||
| First half of slice | 53.1 ± 10.6 | 79.0 ± 15.1 | 85.5 ± 7.8 | — | — | — | 348 ± 18.6 | 656 ± 50.0 | 827 ± 61 | |
| [% of calculated total | (4)b | (5)b | (8)b | (8)b | (8)b | (5)b | (38) | |||
| slice cadmium conc.] | [104 ± 2%] | [107 ± 6%] | [101 ± 3%] | [101 ± 3%] | [104 ± 8%] | [100 ± 1%] | [103 ± 5%] | |||
| Second half of slice | 48.0 ± 9.5 | 67.8 ± 4.3 | 83.9 ± 6.0 | — | — | — | 341 ± 27.8 | 618 ± 107 | 818 ± 57.3 | |
| [% of calculated total | (4)b | (5)b | (8)b | (8)b | (8)b | (5)b | (38) | |||
| slice cadmium conc.] | [94 ± 4%] | [93 ± 6%] | [99 ± 3%] | [99 ± 3%] | [96 ± 7%] | [99 ± 1%] | [97 ± 5%] | |||
| Cortex of slice | 49.9 ± 4.5 | 82.3 ± 4.9 | 97.0 ± 3.0 | 97.3 ± 5.0 | 177 ± 6.0 | 123 ± 2.0 | 383 ± 14.6 | 731 ± 18.4 | 884 ± 52.1 | |
| [% of calculated total | (4)b | (6)b | (8)b | (8)b | (8)b | (8)b | (8)b | (8)b | (5)b | (63) |
| slice cadmium conc.] | [114 ± 12%] | [113 ± 5%] | [114 ± 5%] | [117 ± 2%] | [114 ± 4%] | [115 ± 7%] | [112 ± 4%] | [115 ± 4%] | [114 ± 3%] | [114 ± 5%] |
| Intermediate of slice | 31.9 ± 13.4 | 59.5 ± 6.8 | 67.0 ± 9.8 | 62.4 ± 8.8 | 130 ± 6.9 | 90.1 ± 12.0 | 274 ± 27.4 | 465 ± 53.2 | 587 ± 39.0 | |
| [% of calculated total | (4)b | (6)b | (8)b | (8)b | (8)b | (8)b | (8)b | (8)b | (5)b | (63) |
| slice cadmium conc.] | [72 ± 24%] | [82 ± 7%] | [78 ± 8%] | [75 ± 11%] | [83 ± 2%] | [84 ± 7%] | [80 ± 5%] | [72 ± 5%] | [76 ± 2%] | [78 ± 9%] |
| Medulla of slice | — | 13.0 ± 4.3 | 14.7 ± 13.8 | 30.1 ± 6.6 | 38.9 ± 20.9 | 33.4 ± 6.4 | 74.5 ± 21.2 | 85.1 ± 32.4 | 164 ± 49.0 | |
| [% of calculated total | (6)b | (6)b | (6)b | (5)b | (6)b | (6)b | (6)b | (4)b | (45) | |
| slice cadmium conc.] | [18 ± 5%] | [17 ± 15%] | [36 ± 9%] | [25 ± 12%] | [33 ± 6%] | [22 ± 5%] | [14 ± 5%] | [21 ± 6%] | [23 ± 11%] | |
| Calculated value for | 44.8 | 71.4 | 80.2 | 70.5 | 145 | 91.8 | 331 | 624 | 774 | |
| homogenate based on | ||||||||||
| analysis of zones | ||||||||||
Sample concentrations of cadmium ranged from 40.9 to 923 μg Cd kg−1 WW for halves of slices, 46.9 to 934 μg Cd kg−1 for cortex, 20.1 to 630 μg Cd kg−1 for intermediate and 1.7 to 206 μg Cd kg−1 for the medulla zone. The Cd concentration in the 1st and 2nd slice halves were relatively similar to the homogenates. However the cadmium concentration in paired halves of slices differ (P = 0.011, paired sign test), indicating that the anatomical difference is sufficient to cause different results in cadmium concentrations in corresponding halves of pig kidney slices. Another explanation may be uneven thickness of slices, cut with a knife. It appears as if a slice might be a good substitute for a homogenate in case of problems of contamination during homogenisation.
The distribution of Cd in the zones (% of calculated total slice concentration) was similar in the individual kidneys and approximately 114% in cortex, 78% in intermediate and 23% in medulla. Thus, compared to cattle kidney, the porcine kidney has a more even distribution of Cd within the kidney. The growing/finishing pigs (No. 11–15) were examined more closely as they constitute the main bulk of animals slaughtered in Sweden. An ANOVA showed that the cadmium concentration differed in the different zones (P<0.001), but that it did not matter from which slice in the kidney the zone sample was taken.
The water and ash content (%) did not differ between halves or zones in pig kidneys (Table 4). Pig renal medulla comprises a smaller part of the kidney (2.4 vs. 12%) and has proportionally higher Cd concentration (23 vs. 10%) than the bovine renal medulla (Tables 4 and 2). Hence, the dilution effect by medulla is of lesser importance in pig kidney than in bovine kidney. Analysis by sampling a whole slice of pig kidney from growing/finishing pigs is reported by Petersson Grawé et al.13 The calculated Cd concentration within pig kidney slices in our study did not differ with position of the slice within the kidney. This is probably due to the small impact of medulla Cd content on the total Cd concentration.
| Slice half | Zone of kidney slice | ||||
|---|---|---|---|---|---|
| First half mean ± s (min–max) | Second half mean ± s (min–max) | Cortex mean ± s (min–max) | Intermediate mean ± s (min–max) | Medulla mean ± s (min–max) | |
| a The medulla was too small to separate from the intermediate zone. | |||||
| Water content | 81 ± 1.0 | 81 ± 4 | 81 ± 1.2 | 81 ± 1.4 | 84 ± 3.0 |
| (%) | (78–83) | (78–82) | (77–83) | (72–88) | (73–88) |
| Ash content | 1.1 ± 0.04 | 1.1 ± 0.04 | 1.1 ± 0.08 | 1.1 ± 0.04 | 1.1 ± 0.58 |
| (%) | (1.0–1.2) | (1.0–1.2) | (0.65–1.3) | (0.97–1.3) | (0–3.2) |
| Relative sample weight | 51 ± 4.9 | 49 ± 4.9 | 63 ± 4.2 | 36 ± 5.9 | 2.4 ± 1.1 |
| (%) | (39–62) | (38–61) | (57–67) | (19–50) | (0.85–5.6) |
| % of total cadmium content | |||||
| 40 kg at slaughter | 54 ± 11 | 46 ± 11 | 76 ± 8.1 | 23 ± 8 | a |
| (40–66) | (34–60) | (65–83) | (17–35) | ||
| Growing/finishing | 53 ± 4.3 | 47 ± 4.3 | 69 ± 5.8 | 31 ± 5.7 | 0.59 ± 0.27 |
| (45–60) | (40–55) | (59–87) | (13–41) | (0.42–1.3) | |
| Sows | 51 ± 5.2 | 49 ± 5.2 | 75 ± 5.6 | 25 ± 5.5 | 0.36 ± 0.28 |
| (41–60) | (40–59) | (58–86) | (14–42) | (0.087–1.2) | |
Fresh vs. frozen
A small but significant decrease in water content was seen in the zones of frozen kidneys (P <0.0001, ANOVA), the ranges do however overlap (Table 5). Cadmium concentration was significantly lower in medulla of the fresh kidney (P<0.0001, Mann-Whitney). The difference in Cd cortex concentration is not statistically significant. Freezing causes cells to rupture, probably resulting in diffusion of cadmium within the kidney, causing a change of the Cd concentration that can be detected in the medulla.| Fresh kidney | Frozen kidney | |||
|---|---|---|---|---|
| Zone | Cd conc. mean ± s (min–max; n) | % Water mean ± s (min–max; n) | Cd conc. mean ± s (min–max; n) | % Water mean ± s (min–max; n) |
| a Significantly different from fresh kidney, P <0.0001. | ||||
| Cortex | 141 ± 40 | 80.8 ± 1.9 | 132 ± 34 | 80.0 ± 1.4a |
| (95.0–208; 24) | (77.8–82.8; 24) | (91–188; 24) | (77.4–81.4; 24) | |
| Intermediate | 94.7 ± 33 | 82.8 ± 1.8 | 94.1 ± 30 | 81.4 ± 1.4a |
| (41.6–189; 24) | (79.4–84.7; 24) | (44.8–138; 24) | (79.1–83.2; 24) | |
| Medulla | 16 ± 32 | 87.0 ± 1.5 | 33.9 ± 12.2a | 85.8 ± 1.6a |
| (1.3–132; 16) | (83.1–89.9; 20) | (21.4–72.5; 17) | (82.5–87.7; 17) | |
Comparison of renal homogenate and cortex Cd concentrations
To compare analysis performed on differently sampled kidneys, factors for concentrations in homogenate vs. cortex has been proposed for humans. Svartengren et al.6 suggested the factor 1.25 to calculate cortex cadmium concentrations from homogenate of human kidney, based on a ratio of 2.7∶1 for cortex∶medulla. Scott et al.7 reports a ratio of 2∶1 for human kidney cortex∶medulla. Livingston5 divided human kidney into eight sections from the outer surface to the renal hilus, sectioning the cortex and the medulla into four layers each. The highest Cd levels were found in the outermost sections with a reduction in the concentration through the cortex towards the hilus and no obvious concentration gradient through the medulla.Using the formula from Koh et al.:14 whole kidney Cd = 0.814 × cortex Cd1.04 (mg kg−1) on our mean value from cortex (0.785 mg kg−1) gives a somewhat higher value for the whole kidney (0.633 mg kg−1), than that determined in the homogenates (0.575 mg kg−1). The most likely explanation for the discrepancy is that the sampling techniques differ. In the study by Koh et al.14 the samples were extracted from the kidney using a titanium tube (diameter 10.3 mm, length 100 mm) after which the medulla and cortex were separated, presumably along the cortico–medullary line, whereas our sample has been extracted from the outer cortex (Fig. 1c). Lücker et al.9 proposed the equation: homogenate = 0.77 × cortex (μg kg−1) for transforming bovine cortex Cd concentrations to homogenate Cd concentrations. Using this on our data gives a Cd concentration of 565 μg kg−1, close to the analysed value (575 μg kg−1) in our homogenates. By the description given by Lücker et al.9 the analysed cortex samples (Cd concentration range 111–1730 μg kg−1) appear to be taken from the same anatomical region as our samples.
Sampling technique
The presented data show that the sampling technique affects the results. This is most evident in cattle kidney due to a larger proportion of medulla and lower Cd levels in the medulla of bovine kidney than in pig kidney. The optimal sampling method depends on the purpose of the investigation. To detect small differences in renal Cd levels, as in the case of biological monitoring of Cd exposure, a standardised sampling of the outer part of cortex seems to be an optimal method. Analysis of whole tissue homogenate might be preferable for assessment of Cd intake from consumption of kidney. For a homogenate to become representative a large enough piece of tissue is required. The diffusion of Cd within the frozen kidney indicates that it is also desirable to use fresh kidneys if possible, in order to detect small changes in Cd concentration in kidney cortex between groups of animals.The legislation on sampling of live animals and animal products within the European Union (EU)24,25 aims at optimising the sampling strategy to detect residues and contaminants. The amount or part of tissue sampled, sample preparation and analytical procedures are however not regulated. Measures to ensure the quality of the analytical results are taken by use of reference and accredited laboratories in the official control programs. However, sampling and sample preparation has to be described to enable comparison of the results. Most scientific publications today contain a description of procedures for quality assurance of the analytical results. A detailed description of sampling methods and how samples have been prepared for analysis is however often lacking. A figure or a couple of sentences are often enough to give a comprehensive picture of the procedure. A good description will make it possible to compare and evaluate results.
Acknowledgements
This study was supported by grants from the Swedish Council for Forestry and Agricultural Research and the Foundation for Strategic Environmental Research (Food 21). The authors thank Seved Helmersson for technical assistance.References
- C. Henrikson, in Textbook of veterinary histology, ed. H.-D. Dellman, Lea & Febiger, Philadelphia, PA, USA, 4th edn., 1993, pp. 194–197. Search PubMed.
- L. Friberg and E. Odeblad, Acta Pathol. Microbiol. Scand., 1957, 41, 96 Search PubMed.
- M. Berlin, L. Hammarström and B. Maunsbach, Acta Radiol., 1964, 2, 345.
- C. Dorian, V. H. Gattone II and C. D. Klaasen, Toxicol. Appl. Pharamcol., 1992, 114, 173 Search PubMed.
- H. D. Livingston, Clin. Chem., 1972, 18, 67 Search PubMed.
- M. Svartengren, C. G. Elinder, L. Friberg and B. Lind, Environ. Res., 1986, 39, 1 CAS.
- R. Scott, E. Aughey, G. S. Fell and M. J. Quinn, Hum. Toxicol., 1987, 6, 111 Search PubMed.
- H. von Schenkel, F. Berschauer and G. Gaus, Landwirtsch. Forsch., 1979, 36, 307 Search PubMed.
- E. Lücker, A. Rosopulo, S. Koberstein and W. Kreuzer, Fresenius’ Z. Anal. Chem., 1987, 329, 31.
- V. Antoniou, N. Zantopoulos and H. Tsoukali-Papadopoulou, Vet. Hum. Toxicol., 1995, 37, 20 Search PubMed.
- D. Z. Doganoc, Food Addit. Contam., 1996, 13, 237 CAS.
- P. R. Fitzgerald, J. Peterson and C. Lue-Hing, Am. J. Vet. Res., 1985, 46, 703 Search PubMed.
- K. Petersson Grawé, T. Thierfelder, L. Jorhem and A. Oskarsson, Sci. Total Environ., 1997, 208, 111 CrossRef.
- T.-S. Koh, P. C. Bansemer and A. B. Frensham, Aust. J. Exp. Agric., 1998, 38, 535 Search PubMed.
- H. L. Kramer, J. W. Steiner and P. J. Vallely, Bull. Environ. Contam. Toxicol., 1983, 30, 588 CAS.
- J. Lee, J. R. Rounce, A. D. Mackay and N. D. Grace, Aust. J. Agric. Res., 1996, 47, 877 Search PubMed.
- G. Vos, J. P. C. Hovens and W. V. Delft, Food Addit. Contam., 1987, 4, 73 CAS.
- Nordic Committee on Food Analysis (NMKL), NMKL-Procedur No. 5, 1997, NMKL Secretary General c/o National Veterinary Institute, P.O. Box 8156, N-0033 Oslo, Norway..
- L. Jorhem, S. Slorach, B. Sundström and B. Ohlin, Food Addit. Contam., 1991, 8, 201 CAS.
- M. López Alonso, J. L. Benedito, M. Miranda, C. Castillo, J. Hernández and R. F. Shore, Sci. Total Environ., 2000, 246, 237 CrossRef CAS.
- E. Lücker, A. Rosopulo and W. Kreuzer, Fresenius’ Z. Anal. Chem., 1987, 328, 370.
- A. Pechová, J. Illek, L. Pavlata, M. Sindelár and D. Horky, Acta Vet. Brno, 1998, 67, 103 Search PubMed.
- A. Lindén, I.-M. Olsson and A. Oskarsson, J. AOAC Int., 1999, 82, 1288 Search PubMed.
- Dir 96/23/EC, Off. J. No. L125, 25/05/96, p. 0010–0032 (C lex 396L0023)..
- Des 98/179/EC, Off. J. No. L065, 23/02/98, p. 0031–0034 (C lex 398D0179)..
Footnote |
| † Electronic Supplementary Information available. See http://www.rsc.org/suppdata/an/b0/b005097k/ |
| This journal is © The Royal Society of Chemistry 2001 |
