An enzyme-linked molecularly imprinted sorbent assay
Ioana
Surugiu
,
Lei
Ye
*,
Ecevit
Yilmaz
,
Anatoli
Dzgoev
,
Bengt
Danielsson
,
Klaus
Mosbach
and
Karsten
Haupt†
Lund University, Department of Pure and Applied Biochemistry, Chemical Center, P.O. Box 124, S-22100, Lund, Sweden.. E-mail: Lei.Ye@tbiokem.lth.se;; Fax: +46 46 2224611;; Tel: +46 46 2229560
First published on 11th January 2000
Abstract
Based on a molecularly imprinted polymer, a competitive binding assay analogous to competitive enzyme immunoassay has been developed. The assay is specific for the herbicide 2,4-dichlorophenoxyacetic acid and uses, for the first time, an enzyme-labelled conjugate as a tracer. The label tobacco peroxidase allowed for colorimetric and chemiluminescence detection. The molecularly imprinted polymer was synthesised in the form of microspheres by precipitation polymerisation. The polymer efficiently and selectively bound the analyte in aqueous solution. Calibration curves were obtained corresponding to analyte concentrations ranging from 40–600 μg mL−1 for the colorimetric assay, and from 1–200 μg mL−1 for chemiluminescence assay.
Antibodies are natural receptor molecules that are routinely utilised as analytical reagents in clinical and research laboratories. One of their most common applications is in immunoassays.1 The many different assay formats that have been developed to date have in common as an important step the specific recognition and binding of an antigen by an antibody, thereby the antigen fits exactly into the antibody’s binding site, whereas other, even closely related compounds are excluded from the site.
Researchers have attempted to replace antibodies with smaller, more stable counterparts, and have also been searching for ways to obtain artificial antibody-like receptors. One technique that is being increasingly adopted for the generation of artificial macromolecular receptors is molecular imprinting of synthetic polymers.2–4 One of the milestones in the development of this technology was the demonstration that imprinted polymer particles can be substituted for antibodies in immunoassays.5
The first molecularly imprinted sorbent assay (MIA) was based on a competitive radioligand-binding measurement. This format is analogous to solid-phase radioimmunoassay, except that the immobilised antibody is replaced with a molecularly imprinted polymer (MIP).5 Other assays developed later have used the same principle.6,7 Unfortunately, radioassays involve the handling of radioactive materials and produce radioactive waste, which are undesirable and therefore make the development of assays based on other labels and detection methods attractive. For alternative competitive MIAs one can, in analogy to immunoassays, imagine different approaches. Fluorescent labels8 or non-related fluorescent probes9 have been suggested, since they were considered the most compatible with MIPs. Enzyme labels, on the other hand, although most common with immunoassays, seemed to be less practical in MIAs for two reasons: First, enzymes often only work in aqueous buffers, whereas the use of many imprinted polymers is restricted to organic solvents. Second, the rather hydrophobic nature and highly cross-linked structure of the polymer limits the access of the large protein molecules to the imprinted binding sites. Thus, the development of a MIP-based ELISA has remained a challenge. However, during the last few years MIPs that perform well in aqueous solvents have been developed,7,10–12 and the problem of binding site accessibility might be circumvented by using, instead of large porous MIP particles, imprinted microspheres that have binding sites at or close to their surface.
In the present paper we demonstrate the feasibility of developing imprinted polymer-based immuno-type assays using an enzyme-labelled antigen. As a model system we have chosen as the selective binder a MIP specific for the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D), which can be used in aqueous buffer,12 and the enzyme tobacco peroxidase for colorimetric and chemiluminescence detection.
Experimental
Materials
Trimethylolpropane trimethacrylate (TRIM), 2,2′-azobis-isobutyronitrile and 4-vinylpyridine (4-VP) were from Merck (Darmstadt, Germany). 2,4-Dichlorophenoxyacetic acid, 2,4-dichlorophenoxybutyric acid (2,4-DB), 4-chlorophenoxyacetic acid (4-CPOAc), phenoxyacetic acid (POAc), luminol, 2,4-dichlorophenoxyacetic acid-carboxy-14C (14C-2,4-D; specific activity 15.7 mCi mmol−1) were obtained from Sigma (St. Louis, MO). Tobacco peroxidase (TOP) from transgenic tobacco plants13 was a gift from Irina G. Gazaryan, Chemical Faculty, Moscow State University, Russia. o-Phenylenediamine dihydrochloride was obtained from Pierce (Rockford, Illinois, USA). All other chemicals were of analytical grade, and solvents were of HPLC quality.The 2,4-D–TOP conjugate was prepared using the periodate oxidation method14 with minor modifications.15 Briefly, TOP was activated with NaIO4, and subsequently coupled with diaminopropane. 2,4-D was converted into the N-hydroxysuccinimide ester with N-hydroxysuccinimide and dicyclohexyl carbodiimide. The activated 2,4-D was allowed to react with the TOP-diaminopropane conjugate, resulting in 2,4-D–TOP. After each step, the products were purified by gel filtration. The hapten/enzyme ratio was estimated to be 8∶1 for the final 2,4-D–TOP conjugate by the trinitrobenzenesulfonic acid method.16
Preparation of molecularly imprinted microspheres
For the preparation of MIP-microspheres, a previously reported polymer recipe12 was adapted and combined with a recently developed protocol for the preparation of MIP microspheres by precipitation polymerisation.17 In brief, for the imprinted polymer, 2 mmol of TRIM, 2 mmol of 4-VP, 10 mmol of 2,4-D (print molecule), and 14 mg of polymerisation initiator 2,2′-azobis-isobutyronitrile were weighed into a glass vessel and dissolved in 40 mL of methanol–H2O (4/1, v/v). The solution was sonicated for 5 min, placed in ice and bubbled with nitrogen for 3 min. Polymerisation was carried out at 60 °C in a water bath for 16 h. The fine polymer particles were collected by centrifugation and washed by incubation in methanol–acetic acid (7/3, v/v) three times, followed by a final wash in acetone, to remove the print molecule. The microspheres were then dried at 40 °C. A control polymer was prepared in the same way but without the addition of 2,4-D.Radioligand binding assays
Radioligand binding studies were performed as described in ref. 12. The MIP microspheres were suspended in 0.1 M phosphate buffer pH 7, containing 0.1% Triton X-100, and appropriate volumes were added into 1.5 mL polypropylene test tubes, followed by the radioligand 14C-2,4-D (260 pmol), varying amounts of a solution of a competing ligand if appropriate, and the phosphate buffer to give a total volume of 1 mL. The samples were incubated on a rocking table for 2 h. After centrifugation at 14000 rpm for 5 min, 500 μL of supernatant was withdrawn and mixed with 10 mL scintillation liquid (Ecoscint O, National Diagnostics, Atlanta, GA), from which radioactivity was measured by liquid scintillation counting on a model 2119 Rackbeta β–radiation counter (LKB Wallac, Sollentuna, Sweden).Colorimetric and chemiluminescence assays
MIP microspheres were incubated with an appropriate concentration of 2,4-D–TOP, varying amounts 2,4-D if appropriate, and the phosphate buffer to give a total volume of 1 mL. The samples were incubated on a rocking table for 1.5 h, after which the particles were removed by centrifugation for 5 min at 14000 rpm. For colorimetric measurements, 100 μL of supernatant was withdrawn and added to 200 μL of colorimetric substrate solution (25 mg o-phenylenediamine dihydrochloride, 3.13 μL H2O2 in 8.33 mL of 44 mM citrate buffer pH 5.5) in a microtiter plate. Measurements were performed using a Multiscan MCC/340 microtiter plate reader (Labsystems, Helsinki, Finland). For chemiluminescence measurements, 200 μL of supernatant were added to 200 μL of chemiluminescence substrate solution. This solution was prepared immediately prior to measurements (4 mg luminol dissolved in 2 mL of 0.1 M NaOH and 8 μL of 30% H2O2 were added to 18 mL of 0.1 M phosphate buffer, pH 7). The light emitted was quantified using a model 1250 luminometer (LKB Wallac, Turku, Finland).Results and discussion
Binding specificity of the molecularly imprinted polymer
In our model system we have used MIP microspheres specific for the herbicide 2,4-dichlorophenoxyacetic acid (Fig. 1). The polymer system was similar to the one described in a previous publication,12 containing 4-VP as the functional monomer, and TRIM (instead of ethyleneglycol dimethacrylate) as the cross-linker. However, instead of a monolithic polymer block, small spherical beads were synthesised using a precipitation polymerisation method.17 This implies dilution of the system, and in order to shift the equilibrium toward complex formation between template and monomers, a larger amount of the print molecule has to be used. The imprinted microspheres had a surface area of 7 m2 g−1 (by BET) and an average diameter of 600 nm (as estimated by SEM). In order to verify that the MIP microspheres are comparable to the ground polymer block in terms of binding capacity and selectivity, radioligand binding analysis was performed. Fig. 2a shows the binding of radiolabelled 2,4-D to the imprinted and control microspheres as a function of polymer concentration. Whereas the imprinted polymer binds the ligand and yields a saturation curve, the non-imprinted control polymer shows only very little binding, which confirms the imprinting effect. Approximately 6 mg of the imprinted polymer is needed to bind 50% of the radioligand, which indicates that the binding capacity of the microspheres is lower than that of the ground block polymer reported previously.12 This is, however, of little concern for equilibrium binding assays. A competitive binding assay showed that binding of the radioligand is effectively inhibited by nonlabelled 2,4-D (Fig. 2b). Moreover, the related compounds 4-CPOAc and 2,4-DB (for structures, see Fig. 1) showed less competition, with cross-reactivities of 25 and 10%, respectively, relative to 2,4-D. These values are even lower than those reported in ref. 12. | ||
| Fig. 1 Structure of the print molecule and the related test compounds. | ||
 | ||
| Fig. 2 (a) Binding of radioligand relative to polymer concentration for the MIP (■) and the control polymer (□). (b) Radioligand displacement curves with unlabelled 2,4-D (■), 2,4-DB (○) and 4-CPOAc (△) as competitors, with 2 mg MIP mL−1. Conditions: 0.1 M sodium phosphate buffer, pH 7, 0.1% Triton X-100. B/B0 is the ratio of the amounts of radioligand bound in the presence and in the absence of displacing ligand. | ||
Enzyme-linked binding assays with colorimetric and chemiluminescence detection
Having established that the MIP microspheres selectively bound 2,4-D, we used the same material to develop binding assays with enzyme-labelled analyte using colorimetric or chemiluminescence detection. Tobacco peroxidase was chosen as the label because it has the advantage that for chemiluminescence detection no enhancer is needed. In addition, the same 2,4-D–TOP conjugate has been successfully employed in an immunoassay for 2,4-D.16 In our experiments, the unbound fraction of the 2,4-D–TOP conjugate was quantified after removal of the MIP by centrifugation.In preliminary experiments, it was established that the minimum amounts of 2,4-D–TOP that could be conveniently detected were 4.4 and 1 ng mL−1 for the colorimetric and chemiluminescence assays, respectively. The optimum concentration of MIP microspheres for each assay was established in titration experiments. Binding to the control polymer was much lower than that to the imprinted polymer in both assays (Fig. 3). The 10–15% non-specific binding that was nevertheless observed is probably due to hydrophobic interactions of the protein with the polymer, which cannot be completely cancelled out by the non-ionic surfactant (0.1% Triton X-100) present in all assays. This was confirmed by binding assays carried out with TOP instead of 2,4-D–TOP. At concentrations of 1 and 4.4 ng mL−1, some non-specific adsorption of TOP was observed, although it was much lower than the specific adsorption of 2,4-D–TOP conjugate. It was found that the non-specifically adsorbed TOP could not be displaced from the MIP by adding 2,4-D.
 | ||
| Fig. 3 Binding of 2,4-D–TOP relative to polymer concentration for the MIP (■) and the control polymer (□) using (a) colorimetric detection with 4.4 ng 2,4-D–TOP mL−1 and (b) chemiluminescence detection with 1 ng 2,4-D–TOP mL−1 in 0.1 M sodium phosphate buffer, pH 7, 0.1% Triton X-100. | ||
We have also performed 2,4-D–TOP binding assays with MIP-particles obtained by grinding of a block polymer. These porous particles have a higher surface area (64 m2 g−1 as compared to 7 m2 g−1) but a similar average particle size (1 μm).12 In these experiments, only slightly increased binding to the imprinted polymer as compared to the control polymer was observed (not illustrated), despite the higher binding capacity of the ground MIP particles for the radiolabelled analyte (see above). This confirms the superiority of imprinted microspheres in assay applications based on chemically labelled analyte, including enzyme labels. We attribute this to the small size and the specific method of preparation of these microspheres, which seems to result in a higher number of binding sites being situated at or close to the particle surface and thus accessible for the conjugate.
In a competitive assay format, free 2,4-D effectively competed with the
2,4-D–TOP conjugate for the binding sites in the MIP microspheres,
whereas with the control microspheres, no competition was observed (Fig. 4). The calibration curves for 2,4-D thus
obtained allowed for quantification of the analyte at concentrations
ranging from 40–600 and 1–200 μg mL−1 using
colorimetric and chemiluminescence detection, respectively. When a higher
concentration of 2,4-D–TOP (20 ng mL−1) was used in
the competitive assays, competition by free 2,4-D was only observed at
concentrations ![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) 100 μg mL−1. With the related
compounds 4-CPOAc and POAc as competitors, no typical sigmoid displacement
curve was obtained, although with CPOAc a slight initial decrease in the
binding of 2,4-D–TOP was observed.
100 μg mL−1. With the related
compounds 4-CPOAc and POAc as competitors, no typical sigmoid displacement
curve was obtained, although with CPOAc a slight initial decrease in the
binding of 2,4-D–TOP was observed.
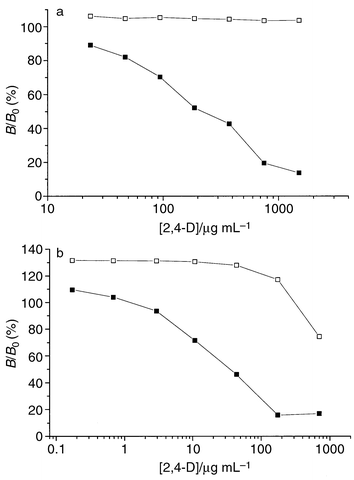 | ||
| Fig. 4 Displacement curves with unlabelled 2,4-D as competitor for the MIP (■) and control polymers (□). B/B0 is the ratio of the amounts of 2,4-D–TOP bound in the presence and in the absence of displacing ligand. (a) Colorimetric detection; conditions: 4.4 ng 2,4-D–TOP mL−1, 6 mg polymer mL−1, 0.1 M sodium phosphate buffer, pH 7, 0.1% Triton X-100. (b) Chemiluminescence detection; conditions: 1 ng 2,4-D–TOP mL−1, 0.5 mg polymer mL−1, 0.1 M sodium phosphate buffer, pH 7, 0.1% Triton X-100. | ||
Conclusions
In the present study we have measured enzyme activity in the supernatants after removal of the MIP, which is different from most immunoassays where the bound analyte–enzyme conjugate is quantified. Research is currently ongoing in our laboratory aiming at developing different assay formats measuring the bound conjugate. However, we have shown for the first time that imprinted polymers can be compatible with enzymes, making colorimetric or chemiluminescence detection possible. ELISA-type binding assays could be developed using enzyme-labelled analyte and a MIP in place of antibodies, which was possible due to the MIP microspheres showing efficient analyte binding in aqueous solvents. Even though our assay is still less sensitive than some recently developed antibody-based assays,16,18 we believe that our findings increase the potential of molecularly imprinted polymers for immunoassay-type applications, and the present or similar approaches could provide useful analytical systems in many instances.Acknowledgements
I.S. acknowledges financial support by the Swedish Research Council for Engineering Sciences.References
- D. S. Hage, Anal. Chem., 1995, 67, 455R CAS.
- G. Wulff, Angew. Chem., Int. Ed. Engl., 1995, 34, 1812 CrossRef CAS.
- B. Sellergren, Trends Anal. Chem., 1997, 16, 310 CrossRef CAS.
- K. Haupt and K. Mosbach, Trends Biotechnol., 1998, 16, 468 CrossRef CAS.
- G. Vlatakis, L. I. Andersson, R. Müller and K. Mosbach, Nature, 1993, 361, 645 CrossRef CAS.
- M. Muldoon and L. H. Stanker, J. Agric. Food Chem., 1995, 43, 1424 CrossRef CAS.
- H. Bengtsson, U. Roos and L. I. Andersson, Anal. Commun., 1997, 34, 233 RSC.
- S. A. Piletsky, E. V. Piletskaya, R. Levi, K. Yano and I. Karube, Anal. Lett., 1997, 30, 445 CAS.
- K. Haupt, A. G. Mayes and K. Mosbach, Anal. Chem., 1998, 70, 3936 CrossRef CAS.
- L. Andersson, R. Müller, G. Vlatakis and K. Mosbach, Proc. Natl. Acad. Sci. USA, 1995, 92, 4788 CAS.
- L. Andersson, Anal. Chem., 1996, 68, 111 CrossRef CAS.
- K. Haupt, A. Dzgoev and K. Mosbach, Anal. Chem., 1998, 70, 628 CrossRef CAS.
- I. G. Gazaryan, Photochem. Photobiol., 1998, 67, 106 Search PubMed.
- P. K. Nakane, J. Histochem. Cytochem., 1974, 22, 1084 Search PubMed.
- B. Dzantiev, Int. J. Environ. Anal. Chem., 1996, 65, 95 Search PubMed.
- A. Dzgoev, I. G. Gazaryan, L. M. Langrimini, K. Ramanathan and B. Danielsson, Anal. Chem., 1999, 71, 5258 CrossRef CAS.
- L. Ye, P. A. G. Cormack and K. Mosbach, Anal. Commun., 1999, 36, 35 RSC.
- A. Dzgoev, M. Mecklenburg, P. O. Larsson and B. Danielsson, Anal. Chem., 1996, 68, 3364 CrossRef CAS.
Footnote |
| † Present address: Université Paris 12 Val de Marne, Faculté des Sciences, IUT de Créteil, CRRET, Avenue du Général de Gaulle, 94010 Créteil Cedex, France. |
| This journal is © The Royal Society of Chemistry 2000 |
