Molecular spectrometry—milestones for the millennium†
R. D. Snook
Department of Instrumentation and Analytical Science, University of Manchester Institute of Science and
Technology, Manchester, UK M60 1QD
First published on UnassignedUnassigned7th January 2000
Abstract
This paper presents an overview of selected aspects of molecular spectrometry in terms of milestone developments from which conclusions about the future development of the subject can be drawn. The paper discusses these in terms of technological, phenomenal and applications milestones. Subjects such as photothermal spectrometry, bioluminescence, and clinical applications of near-infrared spectrometry are seen as exciting developments together with a trend towards miniaturised analytical instrumentation. Longer term predictions about single molecule/particle detection, FT-NIR and terahertz spectrometry are also made.
Introduction
The origins of quantitative molecular spectrometry can be traced back to the work of Bouguer (1729) and Lambert (1760) who described the exponential decrease of transmitted light with increasing sample thickness. The Lambert relationship was later modified by Beer (1852) to yield the now familiar Beer–Lambert law which relates the absorbance of a substance at a particular wavelength to the concentration of the absorbing species in a sample of fixed thickness through the absorptivity, which is a constant for that substance. The realisation of practical quantitative molecular spectrometry, however, did not come about until reliable light sources and detectors in the UV/visible region and the IR region were invented. Thus, the enabling devices in UV/visible spectrophotometry were tungsten and deuterium lamps together with selenium photocells and photomultiplier detectors whilst in the IR region the Nernst glower and globar were developed as sources with bolometer detectors. Developments in grating and prism technology were also of key importance in providing dispersive elements with which spectra could be obtained. Production of these devices can be seen as technological milestones marking the route to spectrometers which we would recognise in the laboratory today, although in the case of the IR instruments dispersive instruments have largely been replaced by FTIR instruments. Such pioneering work in turn has enabled the research of analytical chemists providing powerful spectrometric techniques and methods of quantitative, qualitative and structural analysis in a wide range of chemical and biochemical applications. This might lead us to believe that molecular spectrometry is a mature area of science. However, this is belied by recent advances in technology which have led to a remarkable and diverse range of applications in those fields of science where chemical and biochemical measurements are necessary. New technology has permitted new spectroscopic phenomena to be investigated, none more spectacularly than with the introduction of laser sources which has resulted in a number of non-linear and photothermal spectroscopies hitherto previously unknown as practical techniques. We can consider these as phenomenal milestones leading to techniques which are yet to be fully evaluated in terms of applications. There are also many developments in which an established technique or principle is applied to a new application and we can consider these as application milestones. A good example of the latter is the application of near infrared spectrometry to real-time analysis of clinical and biological samples. Such applications milestones mark the way to the future even though such applications do not always utilise the latest technology.In this paper, therefore, molecular spectrometry is considered in terms of technological, phenomenal and applications milestones observed over recent years and some thoughts are aired about the development of molecular spectrometry in the future. Needless to say that in such a short time as allocated to the lecture from which this paper is derived, the whole subject cannot be reviewed. The following, therefore, is related to my own and closely allied research interests and is not intended to be exhaustive or indeed prescriptive.
Technological milestones
Vibrational spectromentry
Good examples of enabling advances in this respect are Fourier transform infrared spectrometry (FTIR) and the application of lasers as sources for Raman spectrometry. The former opened up the possibility of rapid spectrum acquisition and the consequent ability to carry out kinetic measurements with full spectrum analysis whilst the latter would not be practically possible without lasers, Raman scattering being a weak phenomenon compared with that of absorption. These techniques are complementary and can be used to investigate samples in the solid, liquid or gas phase. Both are based on vibrational spectroscopy and are widely used in industry, providing qualitative or quantitative information about a diverse range of products and processes.1 Indeed, the subject of Raman spectrometry is currently undergoing a renaissance as a result of the use of CCD array detectors, which have facilitated Raman imaging, and notch filters and holographic diffraction gratings which have led to much improved spectral resolution and discrimination against Rayleigh scatter from the laser pump line. The use of near-IR laser sources such as the Nd∶YAG laser (λ = 1.064 μm) has also brought the capability of measuring Raman spectra which are essentially free from visible fluorescence interference. These developments have also opened up the possibility of FT-Raman spectrometry, previously made difficult by the transformation of noise on the pump line into the FT-Raman spectrum with the associated degradation of signal-to-noise ratio. Turning to FTIR spectrometry, perhaps the most exciting development in the IR technology in recent years is the perfection of IR focal plane arrays (FPA) which have facilitated one-dimensional and two-dimensional imaging,2 as can be achieved by the use of CCDs in the visible and near-IR, but throughout the IR region. Different variants of FPA are available which allow the spectral region from 0.4 to 28 μm to be covered as shown in Table 1. The combination of FPA technology, FTIR spectrometry and microscopy yields an extremely powerful imaging technique which is now capable of imaging areas up to 1 × 1 cm with spatial resolution of 100 μm and even higher spatial resolution with smaller image areas. In these systems the modulation of the source by the interferometer is synchronised with the image collection on the FPA, i.e., a series of images is collected as a function of path difference in the optical train of the interferometer. In the transmission mode modulated light from the interferometer is passed down the optical path of a microscope and focused onto the sample which is imaged onto the FPA. The interferometer is normally operated in the step scan mode with an array of images collected at each discrete pathlength difference imposed by the step scan process. Each pixel in the image plane thus contains information which can be recovered as spectral information by subsequent Fourier transformation. Although computationally demanding this type of hyper-spectral imaging provides detailed spectral information at any chosen location in the imaged sample with all of the proven advantages of FTIR. It is not difficult to imagine some of the potential applications of this technology in clinical and medical research and indeed the article cited above2 includes examples of imaging mouse brain tissue and human breast tissue.| Detector type | Operating temperature/K | Wavelength range/μm |
|---|---|---|
| a Adapted from ref. 2. | ||
| Silicon CCD | 273 | 0.38–1.1 |
| InSb | <80 | 0.38–4.6 |
| HgCdTe | <170 | 0.40–2.5 |
| HgCdTe | <55 | 0.40–12 |
| InGaSb | <55 | 0.40–10 |
| Microbolometers | 273 | 8.0–14 |
| SiAs | <12 | 2.0–28 |
FTIR-photoacoustic spectrometry
Another exciting application of FTIR spectrometry is in the area of photoacoustic spectrometry (PAS), enabling acceptable signal-to-noise ratios to be obtained with full spectral coverage. An extremely attractive feature of PAS is the ability to carry out depth profiling of solid materials, provided that the sample is sufficiently transparent to illuminating IR radiation. The principle behind the measurement is the way the interferometer of the FTIR encodes each spectral wavenumber at a different modulation frequency to produce an interferogram from which the spectrum is subsequently reconstructed using the Fourier transform. Therefore, different wavelengths in the spectrum will have different thermal diffusion lengths, μl, in a photoacoustic experiment. The reason for this is that μl depends on the square root of the inverse frequency through the well known equation:3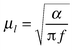 | (1) |
An interpretation of μl is that it is the maximum depth from which a thermal signal can be observed after absorption of optical radiation (assuming that the thermal diffusion length is less than the optical depth). As each wavelength has a different modulation frequency as a result of the multiplexing properties of the interferometer, the diffusion length will vary continuously across the spectrum. For depth-resolved studies by PAS this has often been seen as a disadvantage. However, it may be turned to an advantage, for example in diffusion studies. If the species of interest is diffusing through the sample from below the surface then spectral features corresponding to greater probed depth will appear in time before those at lesser probed depths, i.e., spectral features at one end of the IR absorption spectrum will appear before those at the other. This offers intriguing possibilities in the future for direct measurements of mass transport of chemicals through membranes and thick films. Knowledge of two fixed thermal diffusion lengths at two different absorption wavelengths of the diffusing substance should permit a direct measurement of the diffusion coefficient or mass transport simply by the appearance of the relevant spectral features at different times (assuming that the diffusing species has absorption species spread across the IR spectrum of similar absorption coefficients). Depth information can also be retrieved by recording the phase information inherent in the PAS signal obtained by FTIR-PAS. The phase lag in the PAS signal depends on the depth from which the signal originates in the sample and thus phase angle spectra recorded.
An alternative modality for FTIR-PAS depth profiling is step scanning, whereby the moving mirror is displaced through discrete distances, stopped and data collected at each location. To do this effectively the position of the mirror must be accurately known; this is achieved by dithering the mirror with a known frequency between 25 and 400 Hz and monitoring the resultant interference fringes produced by passage of the reference laser beam through the interferometer. Step scanning has serendipitously given the PAS specialist a potentially powerful tool with which depth profiling can be achieved. Because the output beam of the interferometer is now modulated at a fixed modulation frequency, the thermal diffusion length remains the same at all wavelengths, unlike the dynamic scanning case whereby the thermal diffusion length is directly determined by the wavelength or wavenumber of each spectral component absorbed by the sample. Also, because a single modulation frequency is applied to all wavelengths it is easy to extract the relative photoacoustic phase signal using a lock-in amplifier. First exploited by Palmer et al.,3 step scan FTIR-PAS has recently led to a novel application by Jiang4 in which heterogeneity of single particles and fibres is determined using a step scanning FTIR-PAS micro-sampling technique and phase measurements for signal recovery. The investigation led to depth information concerning oil-coated polymer beads (150 μm diameter) and gel-coated human hair. With the drive in life sciences to develop single cell analysis techniques this could be a milestone development in photoacoustic spectrometry. In our own work we have applied the step scan technique to the measurement of penetration of dimethyl sulfoxide through skin5 by recording phase angle spectra. The maximum probed depth in a sample of epidermis was 20 μm and the practical minimum probed depth was 3 μm. Further studies currently underway are concerned with the penetration of nitroglycerin (an angina treatment) through skin and skin mimetics using both dynamic scanning and step scanning techniques.
Millimetre and sub-millimetre spectrometry
Other examples of advances in enabling technology abound in different regions of the spectrum, for example the introduction of ever higher frequency microwave diode sources (>45 GHz) for communications has spilt over into microwave spectrometry leading to more sensitivity and selectivity for the detection of rotationally active molecules in the gas phase. For example, quantitative millimetre wavelength spectrometry at 60 GHz has been successfully applied by Alder and Baker6 to obtain the response curves of oxygen in carbon dioxide and nitrogen. They used a MMW Fabry–Perot cavity spectrometer to measure oxygen absorption at the 60.306 GHz transition. Using a Gunn oscillator working at this frequency (phase locked with the output of a 12.04 GHz YIG oscillator) the source frequency was scanned across the oxygen spectral line in steps of 50 Hz to obtain the spectrum. The optimum gas pressure was found to be 100 mTorr and the detection limit to be of the order of 1% pressure fraction of oxygen although the authors claimed that this could be improved to 0.05% by off-line data smoothing. There appeared to be no difference in sensitivity between mixtures of oxygen in nitrogen and oxygen in carbon dioxide.Another emerging field is terahertz spectrometry (30 μm–1 mm wavelength) made possible by new optical methods of generating these wavelengths using non-linear crystals, new laser sources and detectors.7 This region of the electromagnetic spectrum was under-utilised because of the previous absence of intense sources and sensitive detectors; with a new generation of devices we are seeing the emergence of practical terahertz spectrometry. Terahertz pulses can be generated using photoconductive switches made of undoped semiconductors such as GaAs, InP and Si which are normally insulating in the off state. Exposure to a femtosecond laser pulse injects electrons and holes into the conductance and valance bands of the semiconductor. With the semiconductor integrated into a transmission line current, transitions of ca. 0.5 ps duration are produced. In an antenna structure these current transients radiate efficiently into the structure and into surrounding air in a wave packet with frequency components around a centre frequency of about 1 THz. Several gases and polar liquids such as water absorb in this region, whereas dry non-metallic materials such as plastics and paper are transparent, which has led to the potential for imaging applications in the security industry8 and clinical and medical sciences
Phenomenal milestones
Apart from technological milestones there are phenomenal milestones, i.e., based on phenomena, often but not exclusively discovered with the introduction of new technology. For example, there is a new class of non-linear spectroscopies which have been brought about by the introduction of lasers as powerful, coherent and monochromatic light sources.Non-linear spectroscopy and thermal lensing
A familiar technique is coherent anti-Stokes Raman spectroscopy (CARS) which is used widely in combustion research, but a lesser known but very similar technique is degenerate four wave mixing (DFWM) in which two forward propagating wavelength degenerate beams are mixed in an absorbing medium to form a diffractive element in the medium with the result that a third forward propagating probe beam generates a fourth counter-propagating beam the intensity of which depends on the absorption coefficient of the medium. Whilst this technique has been of somewhat academic interest some very practical applications have recently been reported including the use of DFWM as a detector for capillary column chromatography.9 In this experiment a laser beam (Ar ion; 1.2 W at λ = 514.5 nm) is split into two pump beams, E1 and E2, which are then focused and recombined at a small angle in the capillary column which forms an interference pattern. On absorption of light by an absorbing species passing through the column a thermally-induced phase grating is produced which results in two diffracted beams, E3 and E4, which emerge in defined directions. The intensity of beam E3 is determined by a number of parameters which include the pump beam intensities, the absorption coefficient of the analyte component, the temperature coefficient of refractive index of the solvent and the efficiency for conversion of absorbed photons into heat. The intensity is inversely proportional to the thermal conductivity of the solvent and the sine of the angle between the two pump beams. In their work, de Beer et al.9 have carefully evaluated the effect of these parameters to produce a detector with a limit of detection (LOD) of 1 × 10−7 M for 1-amino-9,10-anthraquinone which has a molar absorption coefficient of 2000 M−1 cm−1 at 514 nm. This LOD represents an improvement of 50-fold over conventional absorption detection.An attractive feature of DFWM as with other laser-based techniques, e.g., laser-induced fluorescence, is that extremely small volumes of solution can be probed, typically of the order of 100 pL.
Another example of a laser-based phenomenon can be found in thermal lensing, first noticed by physicists as an undesirable effect in laser cavities, which has now been developed as a sensitive analytical spectroscopic technique for trace analysis yielding detection limits orders of magnitude better than UV/visible spectrophotometry10 and high volumetric resolution of the order of picolitres. The technique has also been used to study optical and thermal properties of solutions and solids, to determine parameters such as the temperature coefficient of refractive index,11,12 and absolute quantum yields.13,14 Accordingly, the theoretical models describing the phenomenon and application of thermal lens spectrometry (TLS)15,16 have been developed in parallel with experimental techniques, providing firm foundations for future development of the technique.
In our own work we have been particularly interested in the technique because of the low detection limits which are achievable using spectrophotometric reagents. For example, the TLS determination of aluminium using two different reagents was demonstrated in our early work.17 The best reagent was Chrome Azurol S (CAS) in cetylpyridinium chloride. The Al–CAS complex absorbs strongly at the source laser wavelength (Kr ion; λ = 647 nm) whereas the uncomplexed reagent does not which is an ideal situation for thermal lensing as the detection limit is determined by the noise on the blank, i.e., the thermal lens signal of the uncomplexed reagent. Thus, a detection limit of the order of 10−7 g L−1 Al was determined with a dynamic concentration range of two orders of magnitude. Combined with the ability to probe small volumes the technique has potential for analysis in the interfacial region, for example, at or near electrode surfaces and as a sensitive detector in capillary chromatography, bringing similar benefits to DWFM as mentioned above. It is, however, likely to remain a laboratory diagnostic tool rather than a routine analysis tool.
Applications milestones
Sometimes in analytical science research we study a phenomenon simply because it is there although we are most interested when there is a practical outcome to the research or at least a hint of a practical outcome! Our ability to see potential applications, of technological breakthroughs or phenomenal developments, however, very much depends on our scientific background and environment, i.e., that which we are familiar and comfortable with, yet many recent advances in analytical science are being driven by requirements in other scientific fields where we are less familiar with the terrain. For example, the biotechnology and pharmaceutical industries have generated extreme requirements on rapid throughput analysis techniques such as flow cytometry and analysis techniques for combinatorial synthesis procedures whereas clinical and medical requirements extend to requiring real-time functional analysis and imaging techniques. Many potential solutions to these analysis requirements involve molecular spectrometric techniques. For example, fluorescence spectrometry can be applied to flow cytometry18 and indeed for the detection of fluorescence from single cells in optical trapping experiments using optical tweezers or optical levitation.19Bioluminescence
Equally exciting developments in fluorescence spectrometry applied to the life sciences include the incorporation of green fluorescence protein (GFP) into yeast to provide a genotoxicity sensor.20 The gene for GFP is engineered into a plasmid with a copy of the yeast’s own RAD54 gene. This gene is expressed when DNA damage occurs; since the GFP gene is next to the RAD54 gene it is also expressed. When challenged with DNA damaging agents (which may be mutagens, carcinogens or teratogens), GFP is produced which can be detected selectively by excitation at a wavelength of 488 nm and observing emission between 510 and 520 nm. Discrimination against the natural cellular autofluorescence can be achieved by the use of fluorescence polarisation techniques.Toxicity testing of water supplies can also be carried out using non-genetically engineered organisms, the classic technique being based on the effect of toxic compounds on the photoluminescent bacteria Vibrio fischeri or Photobacterium phosphoreum. Traditionally, these techniques have relied on serial dilution techniques and measurement of the light output of a known concentration of bacteria at pre-determined times after challenge with a toxic compound. In our own laboratories, however, we have developed an on-line kinetic method of measurement which permits rapid determination of relative toxicities of compounds from the background-corrected decay curves of light output.21 The kinetic approach also has the potential for development into a simple dipstick type sensor, work which is currently underway in this department.
A major emerging theme is the application of molecular spectroscopy to clinical and medical diagnostics and functional analysis. Thus, the rise of confocal fluorescence microscopy and Raman microscopy has given biochemists and clinical researchers the ability to study tissue sections with high spatial resolution (2.0 μm) and in the case of confocal microscopy the ability to perform optical sectioning and depth profiling. These kinds of measurements are nearly always in vitro and it is one of the challenges of bioanalytical science to be able to carry out these types of measurement in vivo in a manner which provides dynamic information, offering the possibility of studying biochemical function or metabolism.
The emergence of near infrared spectrometry in this area may offer some solutions in this area of endeavour. Traditionally, the near-IR region or overtone region has been relatively under-exploited, but is now set for an explosion of activity as a result of some clinically significant results relating to metabolism.
Near-infrared spectrometry
It is possible in the near-infrared (NIR) to monitor the absorbance of species such as haemoglobin, deoxyhaemoglobin, myoglobin and cytochrome oxidase. In fact, the first reports of haemoglobin measurements in this way were made by Millikan in 193722 using a dual-wavelength device equipped with green and red filters and simple photovoltaic detectors. With this oximeter device Millikan was able to study animal muscle tissue. The modern version of this device uses laser diodes as light sources and silicon diode detectors or CCDs which may be used in continuous-intensity, time-resolved or intensity-modulated mode as described by Delpy and Cope,23 pioneers in this work. Thus, it has become possible to study a range of clinical conditions in real time which relate to these species or their metabolic utilisation. For example, in the clinical work of Thornily et al.24 NIR spectrometry has been used to study tissue viability of transplant organs by measuring changes in haemoglobin, deoxyhaemoglobin and total haemoglobin in the organ. The NIR technique uses light in the spectral region between 600 and 1000 nm provided by diode lasers via optical fibres and is based on the absorption of haemoglobin chromophores in this region. To perform these measurements accurately, Beer’s law is suitably modified to account for tissue scattering of the incident light. Oxidative metabolism and the capacity to re-synthesise ATP appear to be crucial in the return of a transplanted organ to functionality and measurement of the haemoglobin levels is a good indicator of this.Another exciting development in this area is the use of NIR imaging to map metabolic processes in the body non-invasively even though NIR radiation is highly scattered in tissue. The technique has been made viable by advances in mathematical modelling and algorithms to determine the position of absorbing objects in highly scattering media. These models are based on determining photon migration in the scattering medium. Thus, Tromberg et al.25 have proposed that frequency domain photon migration (FDPM) measurements can be used for non-invasive and quantitative measurements of tissue optical properties and physiological states including normal and tumour-containing breast tissue. In a later paper, Bevilacqua et al.26 used spatially resolved reflectance measurements to determine optical properties of biological tissues and applied these to interoperative in vivo measurements of human skull and brain tissue. Such measurements are fundamental to the application of NIR spectrometry to medical diagnosis because they account for attenuation by scattering. Other applications of the technique are legion and include studies of bowel ischaemia, wound healing, muscle function and heart viability. A common feature of all of these measurements is the nature of the instrumentation which normally consists of a laser diode source at the chosen wavelength of absorption. Scattered radiation is detected by either an array of detectors or an optical fibre array for tomographic imaging purposes or a single silicon diode detector for point measurements. Another common feature of these measurements is the need for even more sophisticated models of photon scattering in tissue if the imaging potential of the NIR region of the spectrum is to be realised as a cheaper alternative to current medical imaging modalities such as positron emission spectrometry and magnetic resonance imaging.
Many of the functional measurements made depend on absorption of species such as myoglobin, haemoglobin, deoxyhaemoglobin and cytochrome oxidase and measurements are often made at a single wavelength which can lead to ambiguity as the absorption spectra of these species are broad leading to the potential for spectral interferences. This field of application is wide open, therefore, for multi-wavelength spectral scanning techniques and in this respect we can confidently predict the use of techniques such as Fourier transform-NIR spectrometry and the application of dispersive instruments and CCD detectors. The latter has been made even more likely with the miniaturisation of such spectrometers to the scale whereby they can be incorporated on the end of an optical fibre using waveguide technology and direct interfacing of the CCD array with a PC.
Miniaturisation
Miniaturisation of analytical instrumentation is a leading edge research topic at present which requires detectors suitable for probing small volumes of solutions. The use of lasers as mentioned above (DFWM) achieves this aim although such relatively complicated equipment arrangements are not compatible with the aims of most miniaturisation programmes to produce robust, small and possibly disposable instruments. Although some laser diodes are available which do fit the bill, i.e., robust and small, they are not ideal sources for spectrometry owing to the limited range of wavelengths available. For general miniaturised spectrometry we must look elsewhere, either by literally reducing the size of components or to look at new techniques altogether. One such possibility being developed in our own laboratories is the use of a waveguide technique known as the resonant mirror in conjunction with CCD array detectors. This device can be used to interrogate extremely thin films as is required in miniaturised electrophoretic and isotachophoretic separations. In essence, light from a conventional source (e.g., an LED) is coupled into a waveguiding layer, some of which penetrates the contacting film to be analysed by the evanescent wave phenomenon. The leakage of light from the waveguide in this manner occurs at angles greater than the critical angle. Changes in refractive index or of the contacting film alter the coupling angle back into the waveguide. Using a prism to launch collimated radiation into the waveguide and to extract refracted light back from the waveguide these changes in angle can be recorded as displacement on a linear CCD array. Indeed, this technique was applied in the first report of an integrated waveguide detector and micro-flow channel separation device by Lenny et al.,27 who used electro-osmotic transport to move samples of different refractive index through a channel (50 μm deep × 300 μm width) in contact with the waveguide detector. The source was a red LED and the detector a 1728 pixel CCD linear array showing a sensitivity of 4 × 103 pixels per refractive index unit. It must be remembered, however, that if a broad band source is used rather than an LED a waveguide of this nature is also dispersive, with different wavelengths appearing at different angles owing to the wavelength dependence of refractive index.Absorption spectroscopy of very small samples typical of micro-total analytical systems (μTAS) can thus be performed using evanescent wave techniques. Such techniques have been used for many years to perform spectroscopy of very small samples, but it is only recently that instrumental advances have made the use of such devices routine. The resonant mirror sensor,28 which is a planar waveguide optical sensor, uses frustrated total internal reflection via a prism coupler to couple light into and out of the waveguiding layer. Previously, this type of sensor has been used as the optical transducer for various types of biosensor, by monitoring the change in coupling angle as the surface refractive index changes with analyte binding to the immobilised biochemical. The enhanced intensity of the evanescent field compared with simple total internal reflection devices indicates that the resonant mirror should be very sensitive to absorbing species in the layer immediately adjacent to the waveguide surface. The coupling angle is a function of the thicknesses and refractive indices of the various layers comprising the sensor, including the sample layer above the waveguiding layer. It is also a function of the wavelength of the input light. By providing a white light input over an appropriate range of input angles, it is possible to obtain a spectrum on the detector by a suitable arrangement of polarisers which pass only the light which has been coupled into the waveguide layer.
The future
Although future developments have been implied throughout this paper there are some more esoteric ideas floating about which might come to fruition. Crystal ball gazing is always a risky business but I recall that some techniques previously thought to be untenable, such as single molecule detection,29 now appear to be looking for an application. Similarly, the seemingly futile exercise of levitating single particles in laser beams or electrostatic fields in the 1970s has come of age, being combined with Raman spectrometry30 to provide analysis of single particles. Again, this must have future relevance to the analysis of single biological cells. Recent reports also highlight the possibility of accurate sizing of levitated particles31 by Fourier transformation of vertically polarised Mie scattering patterns to yield a spatial spectrum from which the size parameter can be determined. To be able to do this at the same time as obtaining the Raman or fluorescence spectrum in apparatus similar to that used for flow cytometry (in which individual particles intercept a laser beam) must be a worthy goal and have a use in, for example, combinatorial analysis.Even more esoteric is the notion that we can replace spectrometers and interferometers completely but this is not so far-fetched. Degenerate four wave mixing as described above is in effect such a technique, especially if the pumping laser which provides the interfering beams is replaced with a tuneable laser such that wider spectral coverage can be achieved. Another possibility is the generation of short duration optical pulse trains which contain a broad range of frequencies which could be used for absorption studies. As mentioned above this technology is beginning to emerge in the form of terahertz spectroscopy (300 GHz to 10 THz) using laser pulses of ca. 100 fs duration. Typically, this represents the one millimetre region to the far-IR (30 μm) of the spectrum. Shorter laser pulses, say 5 fs, will result in useable power in the region between 0 and 150 THz (λ = 2.0 μm), thus covering the IR region. Currently, it has been shown, that, with 10–15 fs pulses, generation and detection of 37 THz pulses is achievable,32 so it is only a matter of time before we could see a general purpose spectrometer based on this technique, albeit an expensive one!
Another prediction is the rapid growth of molecular spectrometric techniques in biological and clinical science. I have illustrated one growth area above in the application of NIR spectrometry to clinical measurements. With the successful introduction of suitable models and algorithms to cope with scattering there is no reason why NIR tomography should not offer a modality of body scanning which is complementary with positron emission tomography and magnetic resonance scanning. An obvious development would be to combine techniques such as FTIR in the NIR region with focal plane imaging techniques as outlined above to yield area spectral information in these clinical situations. The ideal would be the ability to measure functional changes over an area of tissue in real or near-real time. However, the existing generation of FTIR-FPA systems take too long to acquire and process images at reasonable spectral and spatial resolution. Typically, such scans take 10 min. To be of real value for functional imaging in, for example, respiratory measurements, image acquisition and processing needs to be achievable in less than 1 s so that several images can be acquired over one cycle of the biochemical change being studied.
There are other trends which I am sure will continue to develop, including the growing use of spectrometry in process control whether in miniaturised analytical systems or conventional on-line analysis systems. Such trends depend on the perspective and perception of the user. As I said in the Introduction the areas mentioned in this paper are not meant to be prescriptive, so I apologise in advance for omitting what may be your favourite area and hope that some of the ideas and techniques discussed in it illustrate that molecular spectrometry has a long and exciting future.
Acknowledgements
The author thanks the organising and scientific committees of SAC 99 for the invitation to present this lecture.References
- J. M. Chalmers and G. Dent, Industrial Analysis with Vibrational Spectroscopy, RSC Analytical Spectroscopy Monographs, Royal Society of Chemistry, London, 1997. Search PubMed.
- P. Colarusso, L. H. Kidder, I. W. Levin, J. C. Fraser, J. F. Arens and E. N. Lewis, Appl. Spectrosc., 1998, 52, 106A Search PubMed.
- R. M. Dittmar, J. L. Chao and R. A. Palmer, Appl. Spectrosc., 1991, 45, 1104 Search PubMed.
- E. Y. Jiang, Appl. Spectrosc., 1999, 53, 583 Search PubMed.
- M. L. Baesso, R. D. Snook and J. J. Andrews, J. Phys. IV, Colloq. C7, 1994, C7–449 Search PubMed.
- J. F. Alder and J. G. Baker, Anal. Chim. Acta, 1998, 367, 245 CrossRef CAS.
- D. M. Mittleman, R. H. Jacobson and M. C. Nuss, J. Select. Top. Quant. Electron., 1996, 2, 679 Search PubMed.
- M. C. Nuss, Circuits Devices, 1995, March, 25. Search PubMed.
- T. de Beer, G. Ph. Hoornweg, P. Besoo, U. A. Th. Brinkman, N. H. Velthorst and C. Gooijer, Appl. Spectrosc., 1999, 53, 595 Search PubMed.
- R. D. Snook and R. D. Lowe, Analyst, 1995, 120, 2051 RSC.
- S. B. Baptista and C. D. Tran, J. Phys. Chem., 1995, 99, 12952 CrossRef.
- S. M. Colcombe, R. D. Lowe and R. D. Snook, Anal. Chim. Acta, 1997, 356, 277 CrossRef CAS.
- D. Magde and J. H. Brannon, J. Phys. Chem., 1979, 83, 696 CrossRef CAS.
- J. Shen and R. D. Snook, Chem. Phys. Lett., 1989, 155, 583 CrossRef CAS.
- S. J. Sheldon, L. V. Knight and J. M. Thorne, Appl. Opt., 1982, 21, 1663 Search PubMed.
- J. Shen, R. D. Lowe and R. D. Snook, Chem. Phys., 1992, 165, 385 CrossRef CAS.
- R. D. Lowe and R. D. Snook, Anal. Chim. Acta, 1991, 250, 95 CrossRef CAS.
- H. M. Shapiro, Practical Flow Cytometry, Wiley–Liss, New York, 3rd edn., 1995. Search PubMed.
- M. Lankers, J. Popp and W. Kiefer, Fresenius’ J. Anal. Chem., 1996, 335, 354.
- A. W. Knight, N. J. Goddard, P. R. Fielden, N. Billinton, M. G. Barker and R. Walmsley, Meas. Sci. Technol., 1999, 10, 211 CrossRef CAS.
- P. R. Fielden, T. McCreedy, R. D. Snook and B. J. Treves Brown, Anal. Commun., 1996, 33, 335 RSC.
- G. A. Millikan, Proc. R. Soc. London, Ser. B, 1937, 129, 218 Search PubMed.
- D. T. Delpy and M. Cope, Philos. Trans. R. Soc. London, Ser. B, 1997, 352, 649 Search PubMed.
- M. S. Thornily, S. Simpkin, E. Balogun, K. Shaw, K. Shurley, K. Burton and C. J. Green, Philos. Trans. R. Soc. London, Ser. B, 1997, 352, 685 Search PubMed.
- B. J. Tromberg, O. Coquoz, J. B. Fishkin, T. Pham, E. R. Anderson, J. B. Butler, M. Cahn, J. D. Gross, V. Venugopalan and D. Pham, Philos. Trans. R. Soc. London, Ser. B, 1997, 352, 661 Search PubMed.
- F. Bevilacqua, D. Piquet, P. Marquet, J. D. Gross, B. J. Tromberg and G. Depeursinge, Appl. Opt., 1999, 38, 4939 Search PubMed.
- J. P. Lenny, N. J. Goddard, J. C. Morey, R. D. Snook and P. R. Fielden, Sens. Actuators B, 1997, 38–39, 212.
- N. J. Goddard, D. Pollard-Knight and C. H. Maule, Analyst, 1994, 119, 583 RSC.
- R. A. Keller, W. Ambrose, P. M. Goodwin, J. H. Jett, J. C. Martin and M. Wu, Appl. Spectrosc., 1996, 50, 12A Search PubMed.
- K. Shaschek, J. Popp and W. Kiefer, J. Raman Spectrosc., 1993, 24, 69.
- B. Steiner, B. Berge, R. Gausmann, J. Rohmann and E. Ruhl, Appl. Opt., 1999, 38, 1523 Search PubMed.
- Q. Wu and X.-C. Zhang, Appl. Phys. Lett., 1997, 71, 1285 CrossRef CAS.
Footnote |
| † Presented at SAC 99, Dublin, Ireland, July 25–30, 1999. |
| This journal is © The Royal Society of Chemistry 2000 |
