Trace level analysis of VOCs and semi-VOCs in aqueous solution using a direct insertion membrane probe and trap and release membrane introduction mass spectrometry
Maria Anita Mendes and Marcos N. Eberlin*
State University of Campinas—UNICAMP, Institute of Chemistry, CP 6154 13083-970, Campinas, SP, Brazil
First published on UnassignedUnassigned26th January 2000
Abstract
A new design for a trap and release membrane introduction mass spectrometry (T&R-MIMS) system using a removable direct insertion membrane probe (DIMP) for combined trace level analysis (low or sub ppb range) of volatile (VOC) and semi-volatile organic compounds (SVOCs) is reported. The system differs from the original T&R-MIMS system (M. Leth and F. R. Lauritsen, Rapid Commun. Mass Spectrom., 1995, 9, 591) as it uses a removable DIMP probe to place a capillary membrane loop inside the ion source block exactly between two parallel filaments. The more versatile DIMP-T&R-MIMS system can be operated, during the trapping step of T&R-MIMS analysis, in the standard MIMS mode for VOC detection; during the T&R-MIMS thermal desorption step needed for SVOC analysis, the system permits faster and more uniform heating of the membrane, thus SVOC sensitivity is improved and memory effects are minimized.
Introduction
Membrane introduction mass spectrometry (MIMS)1 is a recent, fast-growing technique for the analysis of volatile organic compounds (VOCs), especially in aqueous matrixes. From chemical to biochemical and on to physiological monitoring, MIMS has found widespread use; its many advantages include simplicity, speed, high sensitivity, precision, nearly real-time and in situ monitoring.1MIMS benefits from selective transport of VOCs through a hydrophobic membrane, often of silicone polymer; VOCs are transferred frequently without any extraction or pretreatment from the aqueous matrix directly into a mass spectrometer. The membrane also works as an efficient interface between the matrix and the high vacuum of the mass spectrometer. The VOCs migrate from the aqueous solution to the membrane, concentrate in and diffuse through the membrane, and evaporate from the membrane surface directly into the high vacuum ion source region of a mass spectrometer, in which they are ionized and detected normally at trace levels (low ppbs or less).1
For the analysis of semi-volatile organic compounds (SVOCs) and polar VOCs in aqueous matrixes, standard MIMS has been, however, unsatisfactory since the detection limits are often too high to be useful. Several approaches have therefore been proposed to improve MIMS detection limits for SVOCs and concurrently to lower the detection limits for VOCs: a typical approach has been to combine MIMS with a preconcentration (trapping) method, using solid adsorbents,2 cryotrapping,3 purging,4 headspace trapping,5 or analyte ion trapping.6 Ultrathin composite membranes (25–0.5 μm)7 normally reinforced with a microporous support have also been used to reduce transport time through the membrane. Since the analyte permeability is inversely dependent on membrane thickness, the ultrathin membranes alleviate the effect of low diffusivity or low solubility (or both) of SVOCs in silicone. Chemical derivatization of the SVOCs8 or indirect monitoring of a related VOC analyte can also be useful alternatives for trace SVOC analysis by MIMS; recently, for instance, the selective and sensitive quantitation of cyanogenic glycosides in cassava root extracts was performed via hydrolysis and MIMS monitoring of the released ketone.9
But so far the most generally applicable approach for trace SVOC analysis by MIMS has been offered by the trap and release MIMS (T&R-MIMS) technique developed by Leth and Lauritsen and co-workers10 and Matz and Lennemann.11 Poor responses for SVOCs with standard MIMS often result from their inefficient evaporation from the membrane to the gas phase. The original fully-integrated T&R-MIMS system10 is unique since it uses no external trapping: the SVOCs are preconcentrated inside the membrane itself, before fast thermal desorption12 promotes efficient transport of the SVOCs into the gas phase. A tubular membrane, mounted between two 1/16 in stainless-steel tubes, passes straight through the ion source, and a long slit parallel to both the membrane and the filament allows heat radiation from the filament to reach the membrane continuously. An aqueous solution of the SVOCs (at near 0 °C or room temperature since the SVOC’s solubility in the silicone membrane normally decrease with temperature) is flushed typically for 20 min through the membrane inlet, and during this period the SVOCs dissolve in, diffuse through and concentrate inside the membrane. The cooling fluid is then removed by pumping through the lines a plug of air for typically 50 s;10b when the air plug reaches the membrane, the radiant heat from the filament rapidly heats the membrane to more than 300 °C. As a result, the SVOCs dissolved in the membrane evaporate rapidly, and are efficiently transferred to the gas phase. When compared to standard MIMS, responses are improved, typically 50 or more times higher; hence lower detection limits on the low or sub ppb range are obtained.
We now report on a more versatile and advantageous design for a trap and release MIMS system that uses a removable direct insertion membrane probe (DIMP)13 to place a capillary membrane loop inside the ion source block exactly between two parallel filaments. The new, more versatile DIMP-T&R-MIMS system allows combined trace level analysis (low or sub ppb range) of both volatile (VOC) and semi-volatile organic compounds (SVOCs); it promotes, during the thermal desorption step needed for SVOC analysis, uniform and fast membrane heating and efficient ionization. Hence, sensitivity is enhanced and memory effects are reduced.
Experimental
MS detection (scan speed of typically 6 spectra min−1) was performed using 70 eV electron ionization and an Extrel (Pittsburgh, PA) mass spectrometer fitted with a high transmission (3/4 in) quadrupole. The standard ion source was used with just a minor modification: the id of one of the two gas entrance lines was enlarged to 1/2 in (see Fig. 1). The aqueous solutions were prepared in distilled water by serial dilution of 1 mg mL−1 methanol solutions of the analytes. The solutions at room temperature (23 ± 1 °C) were pumped through the system by an eight-roll peristaltic pump at the rate of 2 mL min−1. The capillary membrane was provided by Dow Corning Co. (Silastic Medical-grade tubing) with a wall tickness of 0.022 in, an id of 0.025 in, and an od of 0.047 in. The capillary membrane was expanded by hexane soaking and then fitted to the 1/24 in steel tubes of the DIMP probe; after hexane evaporation, a strong seal was attained.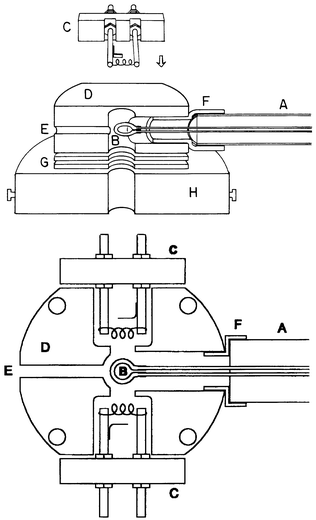 | ||
| Fig. 1 Orthogonal cross-sections of the DIMP-T&R-MIMS system: the highlighted items are: A, DIMP probe; B, capillary membrane loop; C, filaments; D, ion source block; E, CI gas entrance; F, ceramic probe adapter; G, focusing lenses; and H, ion source holder. | ||
Results and discussion
The DIMP-T&R-MIMS system
 | ||
| Fig. 2 DIMP-T&R-MIMS signal profile using selective ion monitoring (SIM) for 500 ppb aqueous solution of β-naphthol. a, Pumping starts; b, air plug is introduced in the system; c, air plug reaches the membrane loop; d, air plug ends; e, first cleaning air plug; and f, second cleaning air plug. | ||
In the original T&R-MIMS system,10 which used a single filament, the ‘dark’ face of the capillary membrane was not sufficiently heated during the desorption step. Responses were therefore not as high and the system was found to be particularly sensitive to undesirable memory effects.10b The uniform heating of the membrane loop provided by the DIMP-T&R-MIMS design results in uniform thermal desorption thus improving sensitivity while reducing memory effects.
 | ||
| Fig. 3 DIMP-T&R-MIMS 70 eV EI mass spectra for 500 ppb aqueous solutions of (a) β-naphthol; (b) benzo(a)pyrene; and (c) nicotine. Note in (c) the ion of m/z 149 corresponding to plasticizers present in the membrane. | ||
Conclusion
The use of a removable direct insertion membrane probe (DIMP) in a trap and release membrane introduction mass spectrometry system is shown to be advantageous. The new DIMP-T&R-MIMS system is more versatile; it can work efficiently and sequentially in both the standard MIMS and T&R-MIMS modes for combined VOC and SVOC analysis, and can be used only when needed, thereby allowing replacement with other MIMS or standard probes, and the standard operation of the mass spectrometer. With the DIMP probe, the capillary membrane loop is placed inside the ion source block exactly between two parallel filaments. Fast and uniform heating of the membrane loop during the T&R-MIMS thermal desorption step is therefore accomplished; sensitivity is improved and memory effects are minimized.Acknowledgements
This work has been supported by the Research Support Foundation of the State of São Paulo (FAPESP) and the Brazilian National Research Council (CNPq).References
- (a) T. Kotiaho, F. R. Lauritsen, T. K. Choudhury and R. G. Cooks, Anal. Chem., 1991, 63, 875A CAS; (b) F. R. Lauritsen and T. Kotiaho, Rev. Anal. Chem., 1996, 237 Search PubMed; (c) N. Srinivasan, R. C. Johnson, N. Kasthurikrishnan, P. Wong and R. G. Cooks, Anal. Chim. Acta, 1997, 350, 257 CrossRef CAS; (d) C. S. Creaser, J. W. Stygall and D. J. R. Weston, Anal. Commun., 1998, 35, 9H RSC; (e) R. C. Johnson, R. G. Cooks, T. M. Allen, M. E. Cisper and P. H. Hemberger, Mass Spectrom. Rev., in the press. Search PubMed.
- (a) J. A. Shoemaker, T. A. Bellar, J. W. Eichelberger and W. L. Budde, J. Chromatogr. Sci., 1993, 31, 279 CAS; (b) A. A. Rivlin, Rapid Commun. Mass Spectrom, 1995, 9, 397 CAS; (c) G. L. Kok, M. E. Cisper and P. H. Hemberger, J. Am. Soc. Mass Spectrom., 1996, 7, 1172 CrossRef CAS.
- M. A. Mendes, R. S. Pimpim, T. Kotiaho and M. N. Eberlin, Anal. Chem., 1996, 68, 3502 CrossRef CAS.
- R. Kostiainen, T. Kotiaho, I. Mattila, M. Ojala and R. A. Ketola, Anal. Chem., 1988, 70, 3028 CrossRef.
- (a) M. A. Mendes, R. Sparrapan and M. N. Eberlin, in Proceedings of the 46th ASMS Conference on Mass Spectrometry and Allied Topics, 1998, p. 750; Search PubMed; (b) M. A. Mendes, R. Sparrapan and M. N. Eberlin, Anal. Chem., in the press. Search PubMed.
- (a) S. Bauer and D. Solyom, Anal Chem., 1994, 66, 4422 CrossRef CAS; (b) M. Soni, S. Bauer, J. W. Amy, P. Wong and R. G. Cooks, Anal Chem., 1995, 67, 1409 CrossRef CAS.
- (a) M. E. Cisper and P. H. Hemberger, Rapid Commun. Mass Spectrom., 1997, 11, 1449 CrossRef CAS; (b) N. Kasthurikrishnan, R. G. Cooks and S. Bauer, Rapid Commun. Mass Spectrom., 1996, 10, 751 CrossRef CAS; (c) R. Alberici, R. Sparrapan, M. N. Eberlin, D. Windmöller and R. Augusti, Anal. Commun., 1999, 36, 221 RSC.
- (a) M. Ojala, R. A. Ketola, V. Virkki, H. Sorsa and T. Kotiaho, Talanta, 1997, 44, 1253 CrossRef CAS; (b) T. K. Choudhury, T. Kotiaho and R. G. Cooks, Talanta, 1992, 39, 1113 CrossRef CAS; (c) R. Alberici, R. Sparrapan, M. N. Eberlin and W. F. Jardim, Anal. Chem., submitted. Search PubMed.
- L. A. B. Moraes, M. N. Eberlin, J. R. Cagnon and L. H. Urbano, Anal. Chem., in press. Search PubMed.
- (a) M. Leth and F. R. Lauritsen, Rapid Commun. Mass Spectrom., 1995, 9, 591 CAS; (b) F. R. Lauritsen and R. A. Ketola, Anal. Chem., 1997, 69, 4917 CrossRef CAS.
- G. Matz and F. Lennemann, J. Chromatogr. A, 1996, 750, 141 CrossRef CAS.
- Laser desorption of SVOCs from the membrane has also been successful, see: (a) M. H. Soni, A. P. Baronavski and S. W. McElvany, Rapid Commun. Mass Spectrom., 1998, 12, 1635 CrossRef CAS; (b) M. H. Soni, J. H. Callahan and S. W. McElvany, Anal. Chem., 1998, 70, 3103 CrossRef CAS.
- (a) M. E. Bier and R. G. Cooks, Anal. Chem., 1987, 59, 597 CrossRef CAS; (b) M. E. Bier, T. Kotiaho and R. G. Cooks, Anal. Chim. Acta, 1990, 231, 175 CrossRef CAS.
- (a) M. A. Mendes, R. S. Pimpim, T. Kotiaho, J. S. Barone and M. N. Eberlin, Quim. Nova, 1996, 19, 480 Search PubMed; (b) R. F. P. Nogueira, R. M. Alberici, M. A. Mendes, W. F. Jardim and M. N. Eberlin, Ind. Eng. Chem. Res.,, 1999, 38, 1754 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2000 |
