DOI:
10.1039/A907856H
(Communication)
Analyst, 2000,
125, 25-27
Liquid chromatography on-chip: progression towards a
μ-total analysis system†
Received 3rd August 1999, Accepted 13th October 1999
First published on UnassignedUnassigned7th January 2000
Abstract
After a brief introduction to the area of miniaturised
analytical separation systems, this paper details the fabrication of
components for a miniaturised liquid chromatography system. To date, inlet
and outlet connection ports, separation μ-channels (20–200 μm
in width, 0.5–10 μm in depth, 15–50 cm in length), and an
intersection for picolitre injection have been etched on a silicon wafer
and then sealed with a Pyrex cover plate on which platinum electrodes for
on-chip detection have been machined. In addition the μ-channel walls
have been chemically modified with n-octyltriethoxysilane and the
reverse phase retention of the drug caffeine was observed using off-chip
injection (20 nl) and UV detection (3 nl flow cell). The on-chip platinum
electrodes have been used for the amperometric detection of phenol. In
addition, using external valving, initial results indicated a successful
mode for a partially integrated on-chip injector which should allow further
progression towards a μ-chromatographic analysis system.
Introduction
In recent years there has been a growing interest and activity in the
area of miniaturised analytical systems, with gas chromatography, capillary
electrophoresis, liquid chromatography and ion chromatography systems all
been miniaturised on silicon or Pyrex substrate with varying degrees of
success.1,2 The microfabrication
technologies employed were initially developed for the microelectronics
industry, where they allow the precise and reproducible construction of
structures ranging from simple channels to pumps with moving parts on
silicon and silicon compatible materials. The main incentives in
miniaturisation include a reduction in the consumption of reagents and
sample, increased analytical performance, shorter analysis time, and
applicability to process and field analysis. The overall goal is
progression towards a μ-total-analysis system, a system whereby chemical
information is periodically transformed into an electronic or optical
signal; analysis is carried out on a micrometer scale using
centimeter-sized glass, silicon or plastic chips.3 While tremendous advances have been made in the
miniaturisation of capillary electrophoretic separation systems, the
challenges of microfabrication of an efficient μ-LC system
remain.1,2,4 By exploiting
micromachining technologies it is relatively easy to fabricate a large
number of columns reproducibly; however there are a number of problems, in
particular the sealing of the μ-channels to allow the mobile phase to be
pumped through the chip at pressures exceeding 2000 psi. Also, there are
many difficulties associated with coupling to external instrumentation
without leakage and without the introduction of dead volumes. In 1990 Manz
et al.5 published research on the
design of an open tubular liquid chromatography column using silicon chip
technology and their results concluded that such a device could be
competitive with an array of chemical sensors. Research at the Laboratory
of the Government Chemists6 showed on-chip
separation with amperometric detection. In 1995 Verpoote et
al.7 slurry packed rectangular channels
on chip using a microfabricated frit to retain the particles. Further
developments were made by Regnier et al.8
whereby collocated monolith support structures were micromachined
within the microchannels, therefore circumventing the need for packing
particles in the column. They concluded that this could provide a route to
highly efficient micro- and nanovolume columns.Despite the contributions of these papers, significant research work is
still necessary in order to achieve the ultimate goal of μ-TAS
incorporating liquid chromatography on-chip. In this paper, we describe the
fabrication of the components of the miniaturised liquid chromatography
system, the reversed phase retention of a drug substance on a coated
μ-channel, the on-chip amperometric detection of phenol, and a mode for
on-chip injection, as a significant step in the progression towards a
μ-chromatographic analysis system.
Experimental
Materials and reagents
Methanol and acetonitrile (HPLC Grade) were obtained from Labscan
(Dublin, Ireland). Caffeine, PEEK tubing, PEEK sleeves and zero dead volume
fittings were obtained from Sigma Aldrich Ltd. (Dublin, Ireland). The
derivatization agent, n-octyltriethoxysilane was purchased from
Fluorochem (Derbyshire, UK). Potassium dihydrogen orthophosphate, disodium
hydrogen orthophosphate, and phenol (analytical grade) were obtained from
BDH (Poole, UK). Sodium chloride was purchased from Merck (Germany). Fused
silica capillary (50 μm id, Type TSP050150) was purchased from Composite
Metals Ltd. (Hallow, Worcester, UK). Gold wire (0.125 mm id) was obtained
from Goodfellow (Cambridge Ltd., UK).Instrumentation
An Ultra Plus micro-pump system (Micro-Tech Scientific, USA) together
with a 20 nl manual injector (Presearch Ltd., UK) was employed for off-chip
pumping and injection. A Shimadzu SPD 6A UV detector (Shimadzu, Japan)
fitted with a 3 nl flow cell (Presearch Ltd.) was used for
spectrophotometric detection. An LC-4B and CC-4 (Biotech Instruments Ltd.,
UK) provided the electronics for amperometric detection. Two platinum
electrodes on-chip and a Ag/AgCl reference electrode, immersed in a small
vial with the external connecting capillary from the chip, constituted the
three-electrode amperometric detection system. Both the UV detection and
amperometric detection outputs were recorded by an HP3392A integrator
(Hewlett-Packard, USA). Connections were realized using PEEK sleeves and
standard LC zero dead volume fittings. For testing on-chip injection,
electropneumatic valves (Ireland Valve & Fittings, Dublin, Ireland)
were employed.Fabrication
The miniaturised liquid chromatography chips were fabricated using
standard photolithography, wet and dry chemical etching, and bonding
techniques described previously.4 Chip layout was designed on a
Sun Sparc workstation at the National Microelectronics Research Centre
(Cork, Ireland). The separation channels and intersection for injection
(Fig. 1) were micromachined on 4 inches
<100> orientation silicon wafers. These channels were isotropically
etched to depths of 0.5–10 μm using the dry etchant
SF6. Using a second photolithography stage and anisotropic
etching, V groove connection ports were micromachined on the same wafers,
into which connecting capillaries could be inserted. The platinum
electrodes (Fig. 2) for electrochemical
detection were micromachined on a Pyrex cover plate. Finally, the patterned
glass silicon wafer was aligned to the patterned Pyrex cover plate and
anodically bonded to form a seal between the silicon and glass (Fig. 3). After diamond sawing to reveal the V
grooves, fused silica capillaries were inserted into the grooves and held
in place using a non-conductive epoxy. Standard LC fittings were then used
to connect the capillaries to the pump, injector, and detector. Finally,
the electrodes were accessed by inserted gold wires (0.125 mm id) into the
grooves and held in place using a conductive epoxy to ensure electric
contact.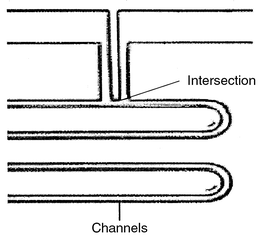 |
| | Fig. 1 Micromachined channels and intersection for on-chip injection. | |
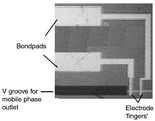 |
| | Fig. 2 Platinum electrodes on-chip. | |
 |
| | Fig. 3 Components of a liquid chromatography system on-chip. | |
Results and discussion
Separation and retention of components with on-chip coated
μ-channels
Previous results from this laboratory have shown the on-chip separation
of the test solutes benzamide and biphenyl in less than two minutes in
μ-channels coated with n-octyltriethoxysilane. The
chromatographic efficiency was low, due to a number of factors including
the volume of injection.4 In this more
recent work the on-chip retention of a pharmaceutical drug substance,
caffeine, is demonstrated. The separation μ-channels (100 μm in
width, 5.3 μm in depth, 16 cm in length) were chemically modified with
an n-octyltriethoxysilane phase (0.5 g of
n-octyltriethoxysilane in 5 ml of toluene) and then interfaced
with the nano-LC system (micro-pump and UV detector with 3 nl flow cell).
The measured flow rate and injection volume were 400 nl
min−1 and 20 nl, respectively, and the off-chip UV
detectior was set at 254 nm. Using a mobile phase of 0.025 mol
l−1 phosphate buffer at pH 2.5 and acetonitrile (98 + 2),
the reversed phase retention of caffeine was obtained (Fig. 4). While the unretained component eluted after
39 s, caffeine was retained and eluted after 111 s. The chromatography of
caffeine, salicylamide, and salicyclic acid has also been studied, but
baseline separation of these components has not yet been achieved. This may
be attributed to the nature of the stationary phase coating on the
μ-channel walls and the relatively large sample loading. To solve this
problem, either a reduction in the injection volume, or improved coating
with sol–gel technology9 or
packing7 may be necessary in the future. The
results, which have been obtained so far, demonstrate the feasibility of a
miniaturised system; however the use of off-chip injection and detection
contribute significantly to band broadening. According to the scaling
factor for nanoscale liquid chromatography, the injection and detection
volumes should be in the low nanoliter to picoliter range for this
system.10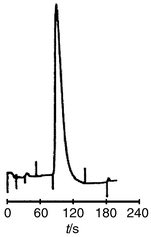 |
| | Fig. 4 Chromatogram showing retention of caffeine (tR = 111
s) on a coated μ-channel. Mobile phase: phosphate buffer pH
2.5–acetonitrile (98 + 2); injection volume: 20 nl; UV detection: 254
nm. | |
On-chip electrochemical detection
In order to reduce detector volume greatly, on-chip detection is
required. Electrochemical detection offers a cheap, small, and sensitive
alternative to the currently employed laser induced fluorescence (LIF)
detection.11 With on-chip microelectrodes,
conductometric detection of sodium nitrate has previously been
shown.4 However, amperometric detection is
also possible with the on-chip electrodes. Amperometric detection was
performed in the three-electrode mode with the upstream electrode as the
working electrode and the electrode closer to the exit as counter
electrode, respectively, together with an external Ag/AgCl reference
electrode. Phenol standards with different concentrations (5–100 mmol
l−1) were injected (20 nl) and amperometrically detected
in an FIA mode with 50 mmol l−1 NaCl as the carrier
stream. The potential applied was +1.0 V. The preliminary results (Fig. 5) show both the feasibility of on-chip
amperometric detection and a good linearity between peak current and
concentration (R = 0.989). As the electrode area is 100 × 80
μm2 each, the corresponding detector volume or cell volume is
as low as 0.052 nl or 52 pl (channel depth is 6.5 μm). This on-chip
amperometric detector will be applied later in conjunction with
chromatography on-chip and improved efficiency is expected. |
| | Fig. 5 Amperometric detection of phenol in a μ-channel with on-chip
microelectrodes. Potential: +1.0 V vs. Ag/AgCl; injection volume:
20 nl; carrier stream: 50 mmol l−1 NaCl; concentrations of
phenol: 10, 25, and 50 mmol l−1. | |
On-chip injection
As with the injection volume, a corresponding reduction in the injection
volume is required; an on-chip injector was therefore designed and tested.
The injection volume is dependent on the μ-channel depth, width, and
also the intersection length, and ranges from the low nanolitres to
picolitres. For testing, the chip was connected to four electropneumatic
valves (Fig. 6),whose opening and closing
were controlled by the application of a voltage, further controlled
via a Labview program. Opening and closing the valves in sequence
can control both the sample flow and carrier flow in the μ-channels.
Initial program sequencing involves filling the μ-channel with mobile
phase, the ‘carrier in valve’ was then closed and sample
solution introduced. After the sample was loaded, the injection was made by
a further valve switching to direct the sample plug through the
μ-channel.This mode of injection has been followed using colored
indicator solutions and well defined sample plugs were successfully
injected. Further research goals involve combining on-chip injection,
separation and electrochemical detection for the integrated μ-liquid
chromatographic analysis system.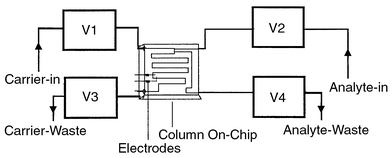 |
| | Fig. 6 Schematic of on-chip injection set-up. | |
Conclusion
Important components for μ-LC, such as injector, separation column,
detector, and connection ports have been fabricated on-chip and tested
separately. The preliminary results have already shown the feasibility of
the endeavor. Even though much work is required to combine all the
functional parts to work simultaneously in a chromatographic mode, the
achievements made so far definitely represent a significant step in the
progression toward the objective of a μ-liquid chromatographic analysis
system.References
- C. S. Effenhauser, Top. Curr. Chem., 1998, 194, 51 Search PubMed.
- F. E. Regnier, B. He, S. Lin and J. Busse, Trends Biotechnol., 1999, 17, 101 CrossRef CAS.
- M. Freemantle,
Chem. Eng. News, 1999, 77(8),
27. Search PubMed.
- M. McEnery,J. Alderman,J. D. Glennon and
S. C. O’Mathuna,
Biomed. Chromatogr., in the
press. Search PubMed.
- A. Manz, Y. Miyahara, J. Miura, Y. Watanabe, H. Miyagi and K. Sato, Sens. Actuators B, 1990, 1, 249 CrossRef.
- S. Cowen and
D. H. Craston,
Proceedings of Micro Total Analysis, University of
Twente, Netherlands, Nov. 1994,
p. 295. Search PubMed.
- G. Ocvirk, E. Verpoorte, A. Manz, M. Grasserbauer and H. M. Widmer, Anal. Methods Instrum., 1995, 2((2), ), 74 Search PubMed.
- B. He, N. Tait and F. E. Regnier, Anal. Chem., 1998, 70, 3790 CrossRef CAS.
- P. Narang and L. A. Colon, J. Chromatogr., A, 1997, 773, 65 CrossRef CAS.
- J. P. Chervet and U. Ursem, Anal. Chem., 1996, 68, 1507 CrossRef CAS.
- L. A. Holland and S. M. Lunte, Anal. Commun., 1998, 35, 1H RSC.
Footnotes |
| † Presented at the SAC 99 Meeting Dublin, Ireland, July
25–30, 1999. |
| ‡ On leave from the Department of Chemistry, Central
South University of Technology, Changsha 410083, China. |
|
| This journal is © The Royal Society of Chemistry 2000 |
Click here to see how this site uses Cookies. View our privacy policy here. 





