Capillary electrophoresis as a practical tool in the study of novel rigid amino alcohols derived from (+)-camphor for catalytic enantioselective addition of organozincs to aldehydes
Susanne K. Wiedmera, Marja-Liisa Riekkolaa, Sylvestre Degnib and Vesa Nevalainenb
aLaboratory of Analytical Chemistry, Department of Chemistry, POB 55, 00014 University of Helsinki, Finland.. Fax: +358-9-19140253
bLaboratory of Organic Chemistry, Department of Chemistry, POB 55, 00014 University of Helsinki, Finland. Fax: +358-9-19140366
First published on UnassignedUnassigned7th January 2000
Abstract
The preparation and catalytic potential of a camphor derivative, 1,7,7-trimethyl-3-(pyrid-2-ylmethyl)bicyclo- [2.2.1]heptan-2-ol, were investigated. The potential of this novel camphor-based 4-amino alcohol to control the addition of organozincs to aldehydes was of particular interest. Several spectroscopic and analytical methods were applied, and major focus was placed on the data obtained by capillary electrophoresis and capillary electrophoresis-electrospray ionization-mass spectrometry; the latter proved to be a fast, reliable and applicable tool in the study of these novel organic synthesis products. The new 4-amino alcohol, 1,7,7-trimethyl-3-(pyrid-2-ylmethyl)bicyclo[2.2.1]heptan-2-ol, was shown to work well as a catalyst in the enantioselective addition of diethylzinc to benzaldehyde.
Aim of the investigation
Fifteen years ago, Oguni and Omi1 reported the capability of chiral 2-amino alcohols to catalyse the enantioselective addition of diethylzinc to benzaldehyde. After this publication, a wide variety of related reactions were reported in the literature.2 2-Amino alcohols are thought to be particularly suitable ligands for this reaction, because both reaction mechanisms (suggested by Kitamura et al.3 and Soai et al.4) lean towards the formation of a zinc chelate. In the zinc chelate, the oxygen and nitrogen of the 2-amino alcohol are bound to a zinc atom of an alkylzinc group, forming a five-membered ring.The catalytic properties of 2-amino alcohols have been widely studied; however, the utility of the related 3- or 4-amino alcohols is less well understood. The structures of the 3- and 4-amino alcohols reported to be useful in this reaction are rather rigid.5,6 In the case of these amino alcohols, the chelating zinc is potentially a member of a six- or seven-membered ring, and the rigidity of the ligand, fixing the orientations of the O and N atoms, stabilizes the chelate sufficiently. As our intention has been to design new cheap ligands for catalytic enantioselective reactions, we decided to study whether the rigidity of the novel camphor-based 4-amino alcohol 1 (Scheme 1) could be capable of controlling the addition of organozincs to aldehydes.
 | ||
| Scheme 1 | ||
According to Richier and coworkers,7–9 a convenient aldol condensation of camphor and benzaldehyde can be carried out in the presence of sodium metal. Therefore, if an enolate of camphor (generated using sodium wire) is allowed to react with pyridine-2-carbaldehyde, enone 3 could be obtained (Scheme 1). Reduction of the olefinic carbon double bond of 3 could then give rise to the ketone 2. In addition, Richier and coworkers7–9 claim that reduction of camphor and related derivatives with metal hydrides favours the formation of exo-alcohols (e.g., compound 1 in Scheme 1). Accordingly, reduction of 2 with lithium aluminium hydride should give rise to the formation of the exo-amino alcohol 1. Interestingly, so far no other studies on the syntheses or structural characterization of 1–3 have to our knowledge been published.
In this work, several analytical techniques were used to study the purity of all the products involved in the various steps of the synthesis (1–3 in Scheme 1), but major focus was placed on studies performed by capillary electrophoresis (CE). CE is an efficient, simple, fast and user-friendly technique, applicable to a vast amount of samples, covering single ions to large macromolecules.10 It has the potential to selectively separate and analyse charged and neutral compounds, as well as enantiomers.11 In the field of chiral CE, a large amount of papers have been published since Gassmann et al.12 reported the first chiral separation performed by CE. Today, 15 years later, many excellent publications on chiral CE are already available, among which can be mentioned the most recent, a review by Chankvetadze.13
In order to obtain charged compounds, acidic buffers were applied in the CE study of compounds 1–3. At pH values higher than 7, no protonation of the pyridine nitrogen occurs; hence, the uncharged compounds co-migrated with the electro-osmotic flow. Because the pure diastereomer of the final product, i.e., 1,7,7-trimethyl-3-(pyrid-2-ylmethyl)bicyclo[2.2.1]heptan-2-ol, is needed for the formation of the zinc chelate, CE was applied to ensure the chiral purity of the synthesis products. For some of the compounds, the diastereomeric ratio was calculated, which was well in accordance with results obtained by gas chromatography (GC). The identification of the peaks in the electropherogram was confirmed by direct injection into the mass spectrometer (MS) and by on-line CE-electrospray ionization (ESI)-MS.
Description of the experimental procedures
Chemicals
Sodium acetate, ammonium acetate, sodium sulfate, sodium hydrogen sulfite, sodium hydrogen carbonate and benzene were purchased from Merck (Darmstadt, Germany). Sodium hydroxide was from Oy FF Chemicals Ab (Yli-Ii, Finland) and β-cyclodextrin was from Sigma (St. Louis, MI, USA). Ethanol was from Primalco Ltd. (Rajamäki, Finland). Methanol and hydrochloric acid were purchased from Mallinckrodt Baker B.V. (Deventer, Holland). (+)-Camphor (natural camphor), diethylzinc (1.1 mol dm−3 toluene solution), 2-pyridine carboxaldehyde and lithium aluminium hydride were purchased from Fluka AG (Buchs, Switzerland). The optical purity of (+)-camphor was about 95%. Magnesium sulfate was purchased from BDH Chemicals Ltd. (Poole, Dorset, UK) and benzaldehyde was from J. T. Baker (Deventes, The Nethertlands; used freshly distilled under argon). Diethyl ether was refluxed with sodium and distilled before use. n-Hexane was distilled from P2O5 and used directly. Distilled water was purified with a Water-I system from Gelman Sciences (Ann Arbor, MI, USA).Preparation of samples and CE buffers
The sample for CE analysis was prepared from stock solutions of the compounds in methanol. The samples for injection were diluted with Water-I water or with buffer solution. The buffers were prepared by mixing appropriate volumes of aqueous 0.1 mol dm−3 or 0.5 mol dm−3 sodium acetate (or ammonium acetate) solutions and adjusting the pH with 0.5 mol dm−3 hydrochloric acid. The chiral buffers were prepared by dissolving β-cyclodextrin in the pH-adjusted buffer. Before electrophoresis, the electrolyte solutions were filtered through 0.45 μm membrane filters and degassed by ultrasonication.Instruments
GC analyses were carried out with a Hewlett-Packard (HP 6890 Series, Hewlett-Packard, Waldbronn, Germany) gas chromatograph, equipped with a flame ionization detector (FID). The column was a 15 m HP-5 (5% diphenyl–95% dimethylsiloxane copolymer) column with a diameter of 320 μm and a film thickness of 0.25 μm. Helium was used as the carrier gas. The GC programme was as follows: from the initial temperature of 35 °C, the temperature was increased to 200 °C by 10 °C min−1.The nuclear magnetic resonance (NMR) 1H and 13C analyses were performed with Varian Gemini 200 (200 MHz), Varian Gemini 2000 (200 MHz) or Varian Inova 300 WB spectrometers (Varian, Palo Alto, CA, USA). The experiments were carried out at room temperature (using the Varian Gemini spectrometers) or at 27 °C (using the Varian Inova spectrometer).
A Waters 600E instrument, equipped with a photodiode array detector, was used in the high-performance liquid chromatography (HPLC) study (Waters, Milford, MA, USA). The column was a CHIRALCEL OB (or OD) column of dimensions 0.46 cm × 25 cm (Daicel Chemical Industries Ltd., Tokyo, Japan). The eluent was n-hexane–isopropanol 95%∶5% (v/v) with a flow rate of 0.6 ml min−1. The injection volume was 20 μl.
Low-resolution (LR) mass spectrometry analysis was performed with a JEOL JMS-SX102 mass spectrometer (Tokyo, Japan). The ionization voltage was 70 V, the temperature of the ionization chamber was maintained at 200 °C and the ion acceleration voltage was 10 kV. The samples were directly injected into the spectrometer.
The flash column chromatographic experiments were performed using silica gel 60, particle size 0.040–0.063 mm (Merck, Darmstadt, Germany), and hexane–ether 75%∶25% (v/v) as the eluent. For the thin layer chromatographic (TLC) analyses, TLC aluminium silica gel 60 F-254 sheets (Merck, Darmstadt, Germany) were adopted. The eluent was as in the flash column chromatographic experiments.
In the preparative thin layer chromatographic analyses, pre-coated preparative liquid chromatography silica gel 60 F-254 plates (Merck, Darmstadt, Germany) were used. Solutions of n-hexane–ether 75%∶25% (v/v) were used as the eluent.
CE studies were carried out with a Beckman P/ACE 2050 instrument equipped with a UV/visible detector and liquid cooling of the capillary (Beckman Instruments, Fullerton, CA, USA) and with a Hewlett-Packard (HP 3D-CE) instrument equipped with a diode array detector and an air cooling device for the capillary (Hewlett-Packard, Avondale, PA, USA). The Hewlett-Packard (HP 3D-CE) instrument was used in the on-line CE-ESI-MS studies. The separations were carried out in uncoated fused silica capillaries (50 μm id, 360 μm od; Composite Metal Services Ltd., Hallow, Worcestershire, UK). The fresh capillary was conditioned for 15 min with 0.1 mol dm−3 sodium hydroxide and 20 min with water. Before each injection, the capillary was rinsed for 3 min with the buffer solution. Sample introduction was performed at 35 or 50 mbar for 5 s. A Jenway pH meter and electrode (Jenway, Felsted, Essex, UK) were used to adjust the pH of the electrolyte solutions.
The mass spectrometer was a Bruker ESQUIRE (Bruker-Franzen Analytik, Bremen, Germany) equipped with an electrospray ionization source (Analytica of Branford, Branford, CT, USA). The spectrometer comprised a hexapole and an ion trap mass analyser. Nitrogen was used as drying gas. The temperature of the drying gas was 250 °C and the flow rate was 2 l min−1. In the CE-ESI-MS on-line studies, the sheath liquid was acidic, containing 0.5% (v/v) acetic acid in methanol. The sheath liquid flow was 3.3 μl min−1. Electrospray voltages were −4 kV for the capillary, −3.5 kV for the end plate and −3 kV for the cylinder. The skimmer was maintained at 15 V, the exit lens at −150 V and voltages at the capillary exit were varied between 40 and 70 V.
Synthesis
Results and discussion
The formation of 3 (Scheme 1) was found to work smoothly to give a total yield of 90%. GC analyses (revealing only one product and a small amount of camphor), as well as the 13C NMR data of the raw product (8 sp2 carbons, see Synthesis), indicated that enone 3 was formed with high stereoselectivity.To further confirm the purity of compound 3 a CE investigation was carried out. CE analysis of product 3, using 0.1 mol dm−3 sodium acetate at pH 5, gave one single peak in the electropherogram, indicating a pure compound. When the pH of the CE buffer was increased to 7, the compound became uncharged and co-migrated with the electro-osmotic flow. Protonation of the pyridine nitrogen is assumed to occur around the same pH for all compounds in Scheme 1; hence, throughout the study, acidic buffers at pH 5 were used in order to ensure charged compounds. Compound 3 was directly injected into the ESI-MS and one major peak (m/z 242), corresponding to protonated ([M + H]+) 3, was obtained.
In the next step, the palladium (Pd/C) catalysed reduction of the enone 3 gave the saturated ketone 2. Quite surprisingly, the CE analysis (buffer as above) of 2 gave two peaks. The sample was spiked with compound 3, but no overlapping of the peaks occurred (absolute migration times: 6.409 min and 7.061 min for compound 2 and 8.770 min for compound 3). Hence, it was obvious that neither of the two peaks from 2 corresponded to compound 3.
On-line CE-ESI-MS analysis of 2 was carried out in order to obtain more specific information about the two peaks (Fig. 1). Since volatile buffers are usually preferred in on-line CE-ESI-MS studies (to prevent the CE buffers from soiling the MS), the sodium ions were replaced by ammonium ions. The signals m/z 246, m/z 228 and m/z 149 could be detected from the faster migrating compound and m/z 244 and m/z 149 from the other compound. Accordingly, the latter compound in the electropherogram corresponds to compound 2 (Mw 243). In the reduction of 3 to 2 it is possible that a small amount of compound 1 is already produced (Scheme 1). This could well explain the extra compound seen in the CE-ESI-MS analysis of 2, i.e., the observed signals (m/z 246 and 228) match with protonated 1 (m/z 246) and its adduct after cleavage of water (m/z 228).
 | ||
| Fig. 1 The electropherogram (A) and mass chromatogram and mass spectra (B) of ligand 2 from the on-line CE-ESI-MS analysis. Peak 1 corresponds to ligand 1 (Mw 245, a by-product formed in the synthesis of ligand 2) and peak 2 corresponds to ligand 2 (Mw 243). Sample concentration: 0.10 g l−1 of 2 in methanol–water, 3%∶97% (v/v). Buffer: 0.02 mol dm−3 ammonium acetate, pH 5 (adjusted with 0.5 mol dm−3 hydrochloric acid). Separation conditions: length of capillary: 25/105 cm (to detector/total); separation voltage: 15 kV; current, 6.2 μA; temperature, 25 °C; injection for 5 s at 50 mbar; detection at 260 nm. The MS inlet capillary exit was maintained at 70 V. Other MS conditions and parameters as in Instruments. | ||
To study the diastereomeric purity of 2, a chiral CE separation, using β-cyclodextrin, was developed. In Fig. 2, the effect of the addition of β-cyclodextrin to the buffer on the CE separation of 2 is shown. Improved peak shapes were obtained by increasing the buffer concentration from 0.02 mol dm−3 to 0.10 mol dm−3; however, since the CE conditions originally had been developed to also work in on-line CE-ESI-MS studies, overall lower concentrations were preferred. With the addition of 0.01 mol dm−3 of β-cyclodextrin [Fig. 2(C)], baseline separation of the diastereomers of compound 2 was achieved (diastereomeric ratio 2∶3), as well as the separation of compound 1 from its dehydrated product. Even though buffers containing cyclodextrins, as well as micelles, can be applied to on-line CE-ESI-MS studies by, for example, using the partial filling technique,14,15 no attempts to analyse the diastereomers by MS were carried out.
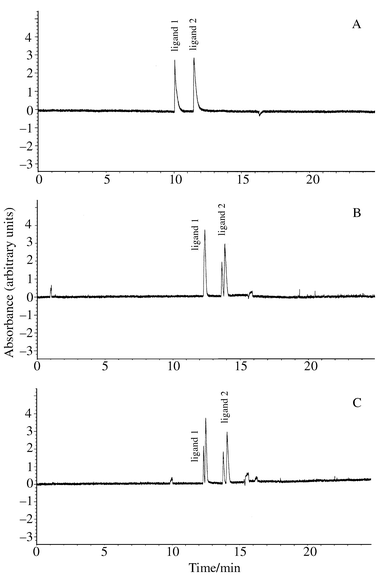 | ||
| Fig. 2 Capillary electrophoretic analysis of compound 2. Sample concentration: 0.15 g l−1 of 2 in methanol–water, 5%∶95% (v/v). Buffers: 0.02 mol dm−3 sodium acetate, pH 5 (A); 0.02 mol dm−3 sodium acetate, pH 5, 0.005 mol dm−3 β-cyclodextrin (B); 0.02 mol dm−3 sodium acetate, pH 5, 0.01 mol dm−3 β-cyclodextrin (C). Separation conditions: length of capillary, 60/68.5 cm (to detector/total); separation voltage, 15 kV; current, 9.2 μA; temperature, 25 °C; injection for 5 s at 35 mbar; detection at 260 nm. | ||
Compound 2 was further reduced, using lithium aluminium hydride, to obtain the amino alcohol 1 in moderate yield. The 13C NMR spectrum of the raw product 1 suggests that a single diastereomer is highly dominating (only one carbinol carbon was observed). 1H NMR data on 1 revealed the coupling constant J(H2, H3) to be 3.9 Hz. In the literature, the coupling constant J(H2-endo, H3-exo) of the corresponding phenyl analogue (phenyl in place of pyridyl) has been reported to be 3.6 Hz.7 This, in addition with the well-known propensity of camphor derivatives to favour an endo-attack of hydride (leading to the formation of a 2-exo alcohol), indicates the configuration of the dominating diastereomer 1 to be H2-endo, H3-exo (Scheme 1). The 1H NMR spectrum of the raw product of 1 confirms the conclusion drawn from the 13C NMR spectrum, i.e., in the raw product a single diastereomer is highly dominating (only one signal, which can be assigned to H2, was observed).
Furthermore, the molecular composition of 1 was confirmed by conventional LR-MS and ESI-MS. A relatively abundant molecular ion corresponding to m/z 245 was observed in the LR-MS spectrum. The ESI-MS spectrum of 1 in Fig. 3 showed both the protonated compound (m/z 246) and the [MH − 18]+ adduct (m/z 228). The signal m/z 268 was the sodium adduct. We soon found out that the ratio of the protonated ion, m/z 246, to m/z 228 (cleavage of water) was highly dependent on the MS capillary exit voltage; by lowering the voltage (80 V to 40 V), higher signals from m/z 246 were observed. The same phenomenon was also observed for m/z 246 and m/z 228 (from compound 1) in the mass spectrum of product 2 [see Fig. 1(b), on-line CE-ESI-MS of 2]. The results from the ESI-MS study, supported by the NMR results, confirm that the elimination of water is a process that mainly happens in the MS (between the MS capillary exit and the skimmer).
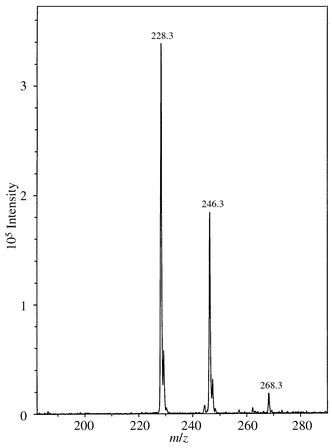 | ||
| Fig. 3 Mass spectrometric off-line analysis (direct injection) of the amino alcohol 1 (Mw 245). Sample concentration: 0.015 g l−1 in acetic acid–methanol, 0.5%∶99.5% (v/v). The MS inlet capillary exit was maintained at 70 V. Other MS conditions and parameters as in Instruments. | ||
In the next step, a CE investigation of compounds 1 and 2 was carried out (Fig. 4). By comparing the absolute migration times of the compounds, it was obvious that the first peak seen in the electropherogram of compound 2 [Fig. 4(B)] was identical with compound 1 [Fig. 4(A)]. Compound 1 was further investigated by CE and, in Fig. 5, it can be seen that, by the addition of methanol to the buffer, the separation of 1 from its dehydrated product was achieved. Methanol is often used in CE to improve the resolution (the electro-osmotic flow is reduced, which results in better resolution at the expense of a longer analysis time).16 Increasing the ionic strength simply by increasing the amount of sodium acetate in the buffer did indeed improve the selectivity; however, baseline separation of compound 1 from its dehydrated product was not achieved until the addition of methanol [Fig. 5(A)]. The lower peak represents the dehydrated form of compound 1. From the ESI-MS spectrum of 1 in Fig. 3, it was evidenced that, in the MS, collision-induced dissociation of water occurs rather easily. Under the acidic CE conditions, a minor peak from the dehydrated product was seen as well; however, the major peak in the electropherogram represents compound 1. If the major compound 1 was the elimination product, then more sp2 carbons should be seen in the 13C NMR spectrum of 1. The addition of β-cyclodextrin to the buffer [Fig. 5(B)] resulted in a single peak from 1 suggesting one dominating diastereomer. Compound 1 had a higher affinity for β-cyclodextrin than its corresponding elimination product (which was also expected considering the chemical structures of the compounds); therefore, the migration order was reversed by the addition of β-cyclodextrin to the buffer.
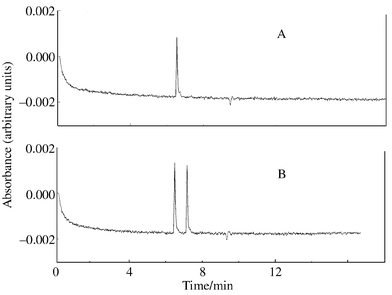 | ||
| Fig. 4 Capillary electrophoretic analysis of compounds 1 (A) and 2 (B). Buffer: 0.1 mol dm−3 sodium acetate, pH 5. Separation conditions: length of capillary, 50/57 cm (to detector/total); separation voltage, 15 kV; current, 44.6 μA; temperature, 25 °C; injection for 5 s at 35 mbar; detection at 260 nm. | ||
 | ||
| Fig. 5 Conventional (A) and chiral (B) CE study of compound 1. Buffers: 0.2 mol dm−3 sodium acetate, pH 5–methanol 80%∶20% (v/v) (A); 0.2 mol dm−3 sodium acetate, pH 5–methanol 80%∶20% (v/v), 0.01 mol dm−3 β-cyclodextrin (B). Sample concentration: 0.3 g l−1 of 1 in methanol–water, 10%∶90% (v/v). Separation conditions: length of capillary, 60/68.5 cm (to detector/total); separation voltage, 15 kV; current, 44.8 μA; temperature, 25 °C; injection for 5 s at 35 mbar; detection at 260 nm. The higher peak represents compound 1, the lower peak its dehydrated product. | ||
In addition to the spectrometric and capillary electrophoretic evidence of the configuration of 1 (Scheme 1), chemical evidence was obtained. If the orientation of the 3-substituent in the precursor 2 was exo (opposite to that shown in Scheme 1), treatment of 2 with a strong base could isomerize the exo-adduct to the more stable endo-epimer (related reactions of the corresponding phenyl derivatives have been reported7). Thus, the amino ketone 2 was treated with sodium methoxide in methanol, but the starting material was found to be fully intact after the treatment. This result supports the conclusion (related to the configuration of H2 and H3 of 1, Scheme 1) drawn on the basis of the data presented above.
When ligand 1 (10 mol%) was used to catalyse the addition of diethylzinc to benzaldehyde at 0 °C, 1-phenylpropan-1-ol was formed with a yield of 75% (isolated using flash column chromatography) and with an enantioselectivity of 81% (determined by chiral HPLC). Therefore, we conclude that camphor-based 4-amino alcohols, such as 1 and its analogues, could be useful for purposes of catalytic enantioselective addition of organozincs to aldehydes. We feel that the results discussed in this report are encouraging, consequently, further studies on the properties of ligand 1, the structural characterization of 1–3 and the development of other substituents of 1 for purposes of catalytic enantioselective synthesis are in progress.
Acknowledgements
The authors are grateful to Seppo Kaltia for operating the NMR and Jorma Matikainen for performing the LR-MS measurements. Further thanks are due to Kai Sinervo for assistance with the ESI-MS measurements.References
- T. Oguni and T. Omi, Tetrahedron Lett., 1984, 25, 2823 CrossRef.
- E. Erdik, Organozinc Reagents in Organic Synthesis, CRC Press, New York, 1996. Search PubMed.
- M. Kitamura, S. Okada, S. Suga and R. Noyori, J. Am. Chem. Soc., 1989, 111, 4028 CrossRef CAS.
- K. Soai, S. Yokoyama and T. Hayasaka, J. Org. Chem., 1991, 56, 4264 CrossRef CAS.
- M. Watanabe, S. Araki, Y. Butsugan and M. Uemura, J. Org. Chem., 1991, 56, 2216.
- B. T. Cho and T. Kim, Tetrahedron Lett., 1994, 35, 4115 CrossRef CAS.
- J. C. Richer and A. Rossi, Can. J. Chem., 1972, 50, 1376 CAS.
- J. C. Richer and C. Lamarre, Can. J. Chem., 1967, 45, 1581 CAS.
- J. C. Richer and R. Clarke, Tetrahedron Lett., 1964, 935 CrossRef CAS.
- High-Performance Capillary Electrophoresis: Theory, Techniques, and Applications, ed. M. G. Khaledi, John Wiley & Sons, New York, 1998. Search PubMed.
- M.-L. Riekkola, S. K. Wiedmer, I. E. Valkó and H. Sirén, J. Chromatogr. A, 1997, 792, 13 CrossRef CAS.
- E. Gassmann, J. E. Kuo and R. N. Zare, Science, 1985, 230, 813 CrossRef CAS.
- B. Chankvetadze, Trends Anal. Chem., 1999, 18, 485 CrossRef CAS.
- L. Valtcheva, M. Jamil, G. Pettersson and S. Hjertén, J. Chromatogr., 1993, 638, 263 CrossRef CAS.
- S. K. Wiedmer, M. Jussila and M.-L. Riekkola, Electrophoresis, 1998, 19, 1711 CAS.
- G. M. Janini, K. C. Chan, J. A. Barnes, G. M. Muschik and H. J. Issaq, Chromatographia, 1993, 35, 497, and references therein. Search PubMed.
| This journal is © The Royal Society of Chemistry 2000 |
