Iron(III) ionophores based on formylsalicylic acid derivatives as sensors for ion-selective electrodes
Mohamed B. Saleh
Chemistry Department, Faculty of Science, Minia University, Minia, Egypt
First published on UnassignedUnassigned7th January 2000
Abstract
Novel iron(III)-selective PVC membrane electrodes based on formylsalicylic acid derivatives were studied. The electrode based on p-chloroaniline-3-formylsalicylic acid as a sensor, containing potassium tetrakis(4-chlorophenyl)borate as a lipophilic salt and o-nitrophenyl octyl ether as a plasticizer, gave the best performance. The electrode exhibits a good Nernstian response for 10−1–5.0 × 10−5 mol l−1 FeCl3 with a slope of 20 mV per decade. It shows a high selectivity for iron(III) in comparison with alkali, alkaline earth and heavy metal ions. The electrode response and selectivity remained almost unchanged for at least 1 month. The effects of plasticizers, membrane supports, lipophilic salts and pH on the potential response of the electrode were also studied. The electrode was successfully applied to the determination of iron(III) contents in some rocks.
In recent decades, many intensive studies on the design and synthesis of highly selective ionophores as sensory molecules for ion-selective electrodes (ISEs) have been reported. In spite of successful progress in the design of highly selective ionophores for various metal ions, there are only a limited number of reports on the development of highly selective ionophores for iron.1–3 Iron is an essential metal and is used for the treatment of anaemia, but excessive intake or overdosing requires a selective analytical method and medication. The only ion-selective membrane sensors described for the determination of iron are based on the use of a coated wire anionic membrane incorporating tetrachloroferrate(III)–tricaprylylmethylammonium chloride,4 a heterogeneous solid-state cationic membrane with tin(IV) arsenate dispersed in epoxy resin5 and a PVC membrane with 1,7-dithia-12-crown-4 as a neutral carrier.6
These sensors, however, suffer from the disadvantages of a low detection limit (>10−4 mol l−1),4,5 a short linear range (10−3–10−5 mol l−1),6 a super-Nernstian response (41 and 56 mV per decade)5,6 and significant interferences from many cations (e.g., Sn2+, Hg2+, Fe2+ and Zn2+).4 Hassad et al.7 constructed a ferroin PVC membrane sensor using tris(1,10-phenanthroline)iron(II) complex–tetraphenylborate (TPB) as an electroactive component plasticized with o-nitrophenyl octyl ether. The electrode showed a low linear emf vs. log [Fe2+] response over the concentration range 10−4–10−6 mol l−1 with a cationic slope of 30 mV per decade. The selectivity coefficients of the ferroin–TPB PVC membrane sensor to Co2+, Cu2+ and Zn2+ (KpotFerroin,B ) were 8.0 × 10−3, 4.0 × 10−2 and 3.0 × 10−2, respectively. An iron(III)-selective electrode based on the iron-containing chalcogenide glass FexSe60Ge28Sb12 (x = 1–10) was studied to optimize the electrode characteristics.8 These sensors showed super-Nernstian slopes of the calibration plots of ≡65 mV per decade. Moreover, an iron(II)–ribonucleic acid (RNA)-based membrane sensor9 exhibited a near-Nernstian slope of 35.5 mV per decade and the selectivity coefficients (KpotFe,B ) of the iron(II)–RNA sensor to all metal ions tested were <10−2.
Formylsalicylic acid derivatives have been reported to form stable metal complexes with different metal ions in the solid state.10,11 Moreover, the iron(III)–salicylic acid complex formed in solution showed a higher sensitivity than other iron(III) complexes with phenolic compounds.12 Recently, the immobilization of formylsalicylic acid has been reported as a selective phase for the extraction of iron(III).13 In this study, formylsalicylic acid derivatives were examined as novel ionophores in iron(III)-selective PVC membrane electrodes. The relative iron(III) response and selectivities with respect to alkali, alkaline earth and heavy metal ions of these sensors in PVC membranes were determined.
Experimental
Materials
Sensor I (3-formylsalicylic acid) was synthesized according to the literature.14,15 Sensors II and III were prepared by Schiff’s base condensation of sensor I with p-toluidine and p-chloroaniline, respectively (Fig. 1). Their structures were confirmed by IR spectrometry and elemental analysis. Poly(vinyl chloride) (PVC), poly(vinyl acetate) (PVA) and polystyrene (PS) were obtained from Aldrich (Milwaukee, WI , USA). The plasticizers o-nitrophenyl octyl ether (o-NPOE) was obtained from Fluka (Buchs, Switzerland), o-nitrophenyl phenyl ether (o-NPPE) from Eastman Kodak (Rochester, NY, USA) and 2-fluoro-2-nitrodiphenyl ether (2-FNDPE ) from Sigma (St. Louis, MO, USA). The lipophilic salts (as membrane additives) used were obtained as follows: sodium tetrakis(4-fluorophenyl)borate (NaTFPB) from Aldrich, sodium tetraphenylborate (NaTPB) from BDH (Poole, Dorset, UK) and potassium tetrakis(4-chlorophenyl)borate (KTClPB) from Fluka. Tetrahydrofuran (THF) was obtained from Aldrich. Analytical reagent grade chlorides and nitrates of the metals were obtained from Aldrich. Doubly distilled water was used throughout. | ||
| Fig. 1 Structures of sensors used in the preparation of iron(III)-selective membrane electrodes. | ||
Electrode preparation
The solvent polymeric membrane electrodes were prepared using established procedures.16–18 Amounts of 3 mg of sensor, 1.5 mg of lipophilic salt, 60 mg of membrane support and 120 mg of plasticizer were dissolved in 2 ml of THF. The resulting mixture was poured into a glass ring with an inner diameter of 30 mm resting on a smooth glass plate and the THF was evaporated at room temperature. Transparent polymeric membranes were obtained with an average thickness of 0.2 mm. A disc of 11 mm diameter was cut out from the polymeric membrane and glued to a PVC tube as described previously.19 The PVC tube with the membrane was then incorporated into an Ag/AgCl wire electrode. After filling with a solution consisting of equal volumes of 10−2 mol l−1 FeCl3 and 10−2 mol l−1 KCl as internal solution, the electrode was conditioned by soaking in 10−2 mol l−1 FeCl3 solution for 1 h and stored in air when not in use. The external reference electrode was a double-junction Ag/AgCl electrode (Cole-Parmer, 7425 N, Chicago, UL, USA).Emf measurements
All potential measurements were made with a Fisher Scientific (Pittsburgh, PA, USA) computer-aided pH-meter (Model 450).The sample solutions were stirred and thermostated at 25 ± 1 °C. Electrode calibration was carried out for solutions of FeCl3 (10−6–10−1 mol l−1) starting from low and proceeding to high concentrations. The series of solutions were obtained by appropriate dilutions. The electrode potential was plotted against the logarthim of iron(III) activity. The activity coefficients used are described in detail elsewhere.20–22 The selectivity of the prepared electrodes was evaluated by the separate solutions method 23,24 using aqueous 0.1 mol l−1 solutions of metal chlorides. The detection limit was determined according to IUPAC recommendations.Determination of iron(III) in rocks
Results and discussion
Effect of plasticizer
The plasticizer used seems to have a significant influence on the response of the electrode. Three plasticizers, o-NPOE, o-NPPE and 2-FNDPE, were examined. The effect of plasticizers on the characteristics of iron (III)-selective electrodes based on sensor III are shown in Fig. 2 and summarized in Table 1. From these data it is clear that the electrode based on sensor III with o-NPOE gives a good response to iron(III) with a Nernstian slope of 20 mV per decade in the concentration range 10−1–5 × 10−5 mol l−1 and a detection limit of 1.5 × 10−5 mol l−1. The observed slope agrees well with the ideal Nernstian slope (19.7 mV per decade at 25 °C), suggesting that the membrane is completely permselective to iron(III), which behaves ideally in this concentration range. With other plasticizers, the performace of the electrode was inferior to that containing o-NPOE. This may be attributed to differences in the sensor mechanism.26 In addition, Table 1 indicates that the electrode with o-NPOE is more selective towards iron(III) than those with o-NPPE and 2-FNDPE plasticizers. All the experimental results described below were obtained with the o-NPOE-plasticized PVC membrane electrodes. | ||
| Fig. 2 Effect of plasticizers on responses of iron(III)-selective electrodes based on sensor III. | ||
| KpotFe,B where B = | |||||
|---|---|---|---|---|---|
| Plasticizer | Na+ | Ca2+ | Ni2+ | Cu2+ | Al3+ |
| o-NPOE | 2.5 × 10−3 | <10−6 | <10−6 | 1.0 × 10−4 | 3.5 × 10−4 |
| o-NPPE | 5.0 × 10−3 | 6.0 × 10−6 | 5.5 × 10−4 | 5.0 × 10−4 | 6.5 × 10−4 |
| 2-FNDPE | 8.0 × 10−3 | 1.5 × 10−5 | 1.0 × 10−5 | 8.5 × 10−4 | 1.5 × 10−3 |
Effect of sensor
The potential response of the iron(III)-selective electrode based on sensor III with the use of o-NPOE as plasticizer is shown in Fig. 3. The electrode exhibits a near-Nernstian response to iron(III) within the concentration range 10−1–5 × 10−5 mol l−1 FeCl3 with a Nernstian slope of 20 mV per decade and a detection limit of 1.5 × 10−5 mol l−1 . As shown in Fig. 3, the responses for sensors I, II were inferior as were the selectivities (Table 2). | ||
| Fig. 3 Potential responses of iron(III)-selective electrodes based on sensors I, II and III. | ||
| KpotFe,B where B = | |||||
|---|---|---|---|---|---|
| Sensor | Na+ | Ca2+ | Ni2+ | Cu2+ | Al3+ |
| I | 8.5 × 10−1 | 1.2 × 10−4 | 4.0 × 10−4 | 1.2 × 10−1 | 1.4 × 10−1 |
| II | 7.0 × 10−1 | 5.6 × 10−4 | 1.6 × 10−3 | 2.4 × 10−1 | 2.2 × 10−1 |
| III | 2.5 × 10−3 | <10−6 | <10−6 | 1.0 × 10−4 | 3.5 × 10−4 |
The results in Fig. 3 and Table 2 indicate that the changes in the structures of sensors I, II and III affect the detection limit , linear regression slope of the electrode response and selectivity. These in turn are expected to be related to the formation constants and distribution coefficients of iron(III) complexes. Hence the introduction of an electron acceptor substituent in the sensor molecule (p-chloroaniline, sensor III) to formylsalicylic acid (sensor I) leads to increase in the selectivity for iron(III) over the cations tested (Table 2). This can be explained on the basis of the increase in the acidity of the phenolic and carboxylic groups representing coordination sites, which in turn is reflected in the high stability of the iron(III) complex formed. On the other hand, introduction of an electron donor substituent ( p-toluidine, sensor II) decreases the selectivity of the electrode towards iron(III). This is due to the expected decrease in the acidity of the sensor. In brief, the membrane response and selectivity can be arranged in terms of sensor molecular structure in the order III > I > II. Therefore, sensor III (p-chloroaniline-3-formylsalicylic acid), which is the best in terms of electrode response and selectivity (Fig. 3 and Table 2), was used in further investigations.
Effect of membrane components
The dependences of the electrode response and selectivity coefficient of the iron(III)-selective PVC membrane electrode on the membrane components are presented in Table 3. The results indicate that the membrane containing only sensor III with o-NPOE (membrane component a) gives a response to iron(III) with a sub-Nernstian slope of 11 mV per decade and a detection limit of 8.0 × 10−4 mol l−1. The response of this electrode is linear in range 10−1–10−3 mol l−1 and the electrode selectivity is low (KpotFe,Cu = 7.0 × 10−3). The theory and experimental results showed clearly that ionic sites should be used not only with neutral ionophores but also with charged ionophores.27| Membrane components | Linearity/mol l−1 | Detection limit/mol l−1 | Slope/mV per decade | KpotFe,Cu |
|---|---|---|---|---|
| (a) sensor III + o-NPOE + PVC | 10−1–10−3 | 8.0 × 10−4 | 11 | 7.0 × 10−3 |
| (b) a + NaTFPB | 10−1–10−3 | 6.0 × 10−4 | 14 | 1.0 × 10−3 |
| (c) a + NaTPB | 10−1–10−4 | 5.0 × 10−5 | 17 | 4.0 × 10−4 |
| (d) a + KTClPB | 10−1–5.0 × 10−5 | 1.5 × 10−5 | 20 | 1.0 × 10−4 |
ISE membranes without ionic additives should be avoided, since otherwise inherent ionic impurities could have a decisive influence on the response characteristics. Moreover, several sensors are known that do not induce any selectivity in membranes in the absence of anionic sites.28,29 The selectivity data for the membrane without anionic site additives are possibly due to interfacial kinetic limitations in the transfer of ions from the sample solution to the membrane phase and vice versa. Lipophilic anionic components can efficiently catalyze this cation transfer.30
Incorporation of NaTFPB or NaTPB as a lipophilic salt with sensor III (membrane components b and c, Table 3) in the membrane phase improves the detection limits (6.0 × 10−4 and 5.0 × 10−5 mol l−1, respectively), increases the slope of the electrode response (14 and 17 mV per decade, respectively) and increases the membrane selectivity (Table 3). It is also obvious from Table 3 that the incorporation of a highly lipophilic salt, KTClPB, with sensor III (membrane component d) in the membrane phase leads to a decrease in the detection limit (1.5 × 10−5 mol l−1), an increase in the slope (20 mV per decade) and an increase in the membrane selectivity compared with NaTFPB or NaTPB. This occurs because its water solubility is lower than that of NaTFPB and NaTPB,31 hence the membrane resistance was lowered and the permselectivity of the membrane for cations improved. In addition, the effect of membrane supports (PVC, PVA and PS) on potential responses of iron (III)-selective electrodes based on sensor III was also studied (Fig. 4), and it is clear that PVC is the best membrane support. Accordingly, the master membrane for the best performance of the iron(III)-selective electrode contains 3 mg of sensor III, 1.5 mg of KTClPB, 120 mg of o-NPOE and 60 mg of PVC.
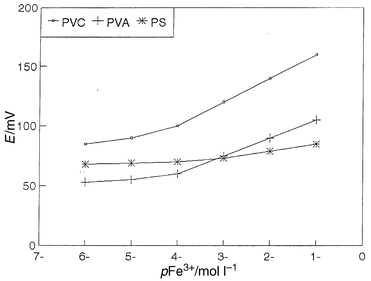 | ||
| Fig. 4 Effect of membrane supports on potential responses of iron(III)- selective electrodes based on sensor III. | ||
Potentiometric response characteristics of the electrode
The dynamic response of the electrode represented by Fig. 3, curve III, was determined by immersion in different concentrations of iron(III) chloride solution. The steady state potential (±0.2 mV) was generally realized within a few minutes, with 95% of the response time being much quicker. The responses are more rapid on proceeding from dilute to concentrated solutions than conversely. The response times are 1 min for concentrated solutions and 2 min for concentrations of <10−5 mol l−1.The stability of the electrode potential was better than ±0.2 mV for short term periods (seconds to minutes) and better than ±1.5 mV for long term periods (hours to days) and the change in the cationic slope was less than 0.5 mV per decade. Drifts (stability and reproducibility) of the electrode potential over a period of 1 month were within 5 mV, but the linear regression slope remained constant to within ±1.0 mV per decade. Hence the detection limit, linear range and selectivity coefficient values remained almost constant for at least 1 month.
Effect of pH
Hydrogen ion concentration has an effect on the electrode potential (Fig. 5) ( adjusted with HCl or NaOH) . Thus, the iron(III)-selective electrode responded only to metal solutions in the pH range 2.1–3.3. Above pH ≈3.3 there is a decrease in the measured potential, probably due to the hydrolysis of iron(III). Moreover, below pH ≈2.1, an electrode potential arises owing to the contribution of the hydrogen ion function of the components TClPB, o-NPOE and sensor III. Hence the experimental results were obtained for sample solutions of pH 2.1–3.3. | ||
| Fig. 5 Effect of pH on the potential responses of iron(III)-selective electrodes based on sensor III: (a) 10−2 and (b) 10−3 mol l−1. | ||
Selectivity
The selectivity of the electrode was first investigated by the mixed solutions method,22,23 but the calculation of the selectivity coefficients is inaccurate, as the membrane is not ideal over the whole measured activity range. Therefore, the potentials obtained using the electrode were measured in a separate solution of iron(III) and of the interfering ion. The values obtained for the selectivity coefficients by the separate solutions method using 0.1 mol l−1 aqueous solutions of the metal chlorides, except for Hg(NO3)2 (10−2 mol l−1), are given in Table 4. From these values, it can be seen that the electrode is characterized by a high selectivity towards iron(III) with respect to alkali metal ions (Li+ , Na+ and K+), alkaline earth metal ions (Mg2+ , Ca2+ , Sr2+ and Ba2+), transition metal ions (Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+ and Hg2+) and other ions (NH4+, Al3+ and Ce3+).| Ion (B) | KpotFe,B | Ion (B) | KpotFe,B |
|---|---|---|---|
| Li+ | 1.0 × 10−3 | Fe2+ | 1.7 × 10−2 |
| Na+ | 2.5 × 10−3 | Co2+ | 1.1 × 10−6 |
| K+ | 6.3 × 10−6 | Ni2+ | <10−6 |
| NH4+ | 1.4 × 10−3 | Cu2+ | 1.0 × 10−4 |
| Mg2+ | <10−6 | Zn2+ | <10−6 |
| Ca2+ | <10−6 | Cd2+ | 1.6 × 10−6 |
| Sr2+ | 2.9 × 10−6 | Hg2+ | 6.3 × 10−3 |
| Ba2+ | <10−6 | Al3+ | 5.4 × 10−4 |
| Mn2+ | <10−6 | Ce3+ | 8.9 × 10−6 |
Comparison of the iron(III)-selective electrode based on sensor III (the present electrode) and previously described electrodes4–9 showed that the proposed electrode exhibits a much better range (10−1–5.0 × 10−5 mol l−1), cationic slope (20 mV per decade) and potentiometric selectivity for Fe3+ over nearly all metal ions tested. The superiority of sensor III over other reported ionophores is attributed to the high stability of its complex with iron(III).
Analytical applications
Determination of iron(III) in some rocks was performed by using the iron(III)-selective electrode. The results were compared with data obtained by a conventional volumetric technique (complexometric EDTA titration) (Table 5). The data showed agreement between the data for complexometric EDTA titration and the iron(III)-selective electrode.| Iron(III) content (% w/w)a | ||
|---|---|---|
| Sample | Iron electrode | Complexometric EDTA titration |
| a Average of three measurements. | ||
| Serpentinite (1) | 4.245 ± 0.06 | 4.368 ± 0.08 |
| Serpentinite (2) | 5.092 ± 0.07 | 5.152 ± 0.08 |
| Metabasalt (1) | 8.305 ± 0.08 | 8.232 ± 0.09 |
| Metabasalt (2) | 6.615 ± 0.06 | 6.552 ± 0.09 |
| Tuff (volcanic rock) | 6.321 ± 0.07 | 6.496 ± 0.08 |
| Meta-tuff | 3.122 ± 0.05 | 3.080 ± 0.07 |
| Meta-andesite | 9.761 ± 0.09 | 9.968 ± 0.15 |
Conclusion
Formylsalicylic acid derivatives can be used for iron(III)-selective electrodes. The optimum membrane components are 3 mg of p-chloroaniline-3-formylsalicylic acid (sensor III), 1.5 mg of KTClPB as a lipophilic salt, 120 mg of o-NPOE as a plasticizer and 60 mg of PVC as a membrane support. The electrode based on sensor III has excellent electrochemical selectivity and sensitivity compared with sensors I and II and previously described electrodes.4–9. The proposed electrode can be used successfully to determine iron(III) contents in some rock materials.Acknowledgement
The author cordially thanks Professor Dr. E. M. Soliman of the Chemistry Department, Faculty of Science, Minia University, for help with the preparation of the sensors.References
- K. Cammann, Working with Ion Selective Electrodes, Springer, Berlin, 1979. Search PubMed.
- D. Ferg, Ion-Sel. Electrode Rev., 1987, 9, 95 Search PubMed.
- R. L. Solsky, Anal. Chem., 1988, 60, 106R CAS.
- R. W. Cattrall and C.-P. Pui, Anal. Chem., 1975, 47, 93 CrossRef CAS.
- P. S. Thind and S. K. Mittal, Bull. Electrochem., 1988, 4(4), 413 Search PubMed; P. S. Thind and S. K. Mittal, Anal. Abstr., 1988,8J, 125. Search PubMed.
- T. Y. Wang and J. S. Shih, J. Chin. Chem. Soc., 1988, 35, 405 Search PubMed.
- S. S. M. Hassan and S. A. M. Marzouk,Talanta, 1994, 41(6), 891. Search PubMed.
- C. E. Koenig and E. W. Grabner,Electroanalysis, 1995, 7(11), 1090. Search PubMed.
- S. S. M. Hassan, W. H. Mahmoud and A. H. M. Othman, Talanta, 1998, 47, 377 CrossRef CAS.
- M. J. Adam and L. D. Hall, Can. J. Chem., 1982, 60, 2229 CAS.
- U. Casellato, D. Fregona, S. Sitran, S. Tamburini and P. A. Vigato, Inorg. Chim. Acta, 1985, 110, 161 CrossRef CAS.
- P. H. Gore and P. J. Newman, Anal. Chim. Acta, 1964, 31, 111 CrossRef CAS.
- M. E. Mahmoud and E. M. Soliman, Talanta, 1997, 44, 15 CrossRef CAS.
- J. C. Duff and E. J. Bills, J. Chem. Soc., 1932, 1987 RSC.
- M. Vidali, U. Casellato, P. A. Vigato, L. Doretti and F. Mada Losso, J. Inorg. Nucl. Chem., 1977, 39, 1985 Search PubMed.
- G. J. Moody, R. B. Oke and J. D. R. Thomas, Analyst, 1970, 95, 910 RSC.
- A. Craggs, G. J. Moody and J. D. R. Thomas, J. Chem. Educ., 1974, 51, 541 Search PubMed.
- S. S. M. Hassan and F. Sh. Tadros, Anal. Chem., 1984, 56, 542 CrossRef CAS.
- M. B. Saleh, Anal. Lett., 1999, 32, 2201 CAS.
- P. C. Meier, D. Ammann, W. E. Morf and W. Simon, in Medical and Biological Applications of Electrochemical Devices, ed. J. Koryta, Wiley, Chichester, 1980, p. 13. Search PubMed.
- P. C. Meier, D. Ammann, H. F. Osswald and W. Simon, Med. Prog. Technol., 1977, 5, 1 Search PubMed.
- M. Linder, K. Toth and E. Pungor, Anal. Chem., 1984, 56, 1127 CrossRef.
- S. S. M. Hassan and G. A. Rechnitz, Anal. Chem., 1986, 58, 1052 CrossRef CAS.
- IUPAC Analytical Chemistry Division, Commission on Analytical Nomenclature, Pure Appl. Chem., 1995, 67, 507.
- E. H. Hansen, C. G. Lamm and J. Ruzicka, Anal. Chim. Acta, 1972, 59, 403 CrossRef CAS.
- E. Pungor and K. Toth, Anal. Proc., 1985, 22, 352 Search PubMed.
- U. Schaller, E. Bakker, U. E. Splchiger and E. Pretsch, Anal. Chem., 1994, 66, 391 CrossRef CAS.
- W. E. Morf, G. Kahr and W. Simon, Anal. Lett., 1974, 7, 9 CAS.
- R. Eugster, P. M. Gehrig, W. E. Morf, U. E. Spichiger and W. Simon, Anal. Chem., 1991, 63, 2285 CrossRef CAS.
- P. M. Gehrig, W. E. Morf, M. Welti, E. Pretsch and W. Simon, Helv. Chim. Acta, 1990, 73, 203 CrossRef CAS.
- D. Ammann, W. E. Morf, P. Anker, P. C. Meier, E. Pretsch and W. Simon, Ion-Sel. Electrode Rev., 1983, 5, 3 Search PubMed.
| This journal is © The Royal Society of Chemistry 2000 |
