The authentication of olive oil on the basis of hydrocarbon concentration and composition†
Lynda Webster*, Pamela Simpson, Aileen M. Shanks and Colin F. Moffat
FRS Marine Laboratory Aberdeen, P.O. Box 101, Victoria Road, Aberdeen, UK AB11 9DB
First published on UnassignedUnassigned7th January 2000
Abstract
Samples of virgin olive oil and refined olive oil were analysed for n-alkanes by gas chromatography with flame ionisation detection to determine if the pattern and composition were oil specific and, therefore, if the hydrocarbon patterns could be used as determinants for assessing adulteration of olive oil. The carbon number profile of the extra virgin olive oil was unique inasmuch as the odd numbered predominance was not limited to two or three n-alkanes but started at tricosane (nC23) and continued to tritriacontane (nC33). The olive oil n-alkane data was added to an existing database that included rapeseed, safflower, sunflower, corn, palm, palm kernel, coconut, groundnut and soyabean oils and analysed by principal component analysis (PCA). Olive oil could clearly be differentiated from the other vegetable oils. PCA also allowed for the distinction of olive oils from different geographical regions. Authentic extra virgin olive oil was adulterated with various amounts of either crude sunflower or crude rapeseed oil, which resulted in adulteration levels between 0.5 and 11% w/w. Using the carbon number profiles alone it was possible to determine adulteration of the extra virgin olive oil with as little as 2.6% crude rapeseed oil or crude sunflower oil. Analysis of the n-alkane pattern by PCA made it possible to identify adulterants at levels as low as 0.5% w/w.
The hydrocarbon composition of edible oils has been studied to a limited extent and the odd numbered carbon long chain predominance is well documented.1–4 These n-alkanes are endogenous to a plant and are thought to be the result of decarboxylation of long chain fatty acids.5,6 Previous published work indicated that it was possible, using the n-alkane pattern and composition and the sum of the individual n-alkanes between pentadecane (nC15) and tritriacontane (nC33), to distinguish between crude and refined oils of different plant origin.7 By using principal component analysis (PCA) and discriminant analysis, the specific patterns have been shown to be significantly different, especially for crude oils.8,9 It may therefore be possible to use the n-alkane profiles as a means of determining olive oil authenticity and any adulteration of olive oil with lower priced oils.
Olive oil is unique among edible oils because of its stability, delicate flavour and reported health benefits.10 It is produced mainly in Mediterranean countries and can be consumed directly without purification. It is expensive and has always been the subject of fraud by mixing with less expensive vegetable oils or else traded as a higher grade product.11,12 In 1993–94, 1829000 tonnes of olive oil were consumed world-wide. Hence it is critical that the authenticity of both crude and refined olive oils can be established. Several recognised international bodies have introduced control measures to ensure standardised quality and classification of the various types of olive oils available.13,14 These standards include limits for the volatile matter, insoluble inmpurities, trace metals and acidity (expressed as a percentage of oleic acid); if necessary, these standards can be used for enforcement purposes. This analysis, currently required for the detection of adulteration of olive oils, is protracted, complex and costly. Criteria for the detection of seed oils include determining the maximum difference between the real and theoretical ECIV42 triglyceride content and also the sterol and stigmastadiene content. A number of alternative techniques for assessing both quality and authenticity have been reported. These include sensory analysis,15 CO2 laser infrared optothermal spectroscopy16 and near infrared spectroscopy.17 The determination of authenticity on the basis of n-alkane composition could provide a cheaper and simpler alternative to the current methods employed and the more recent alternative methods.
There are limited data on the n-alkane composition of olive oils, although published work7 has shown that, for a limited number of olive oils, the n-alkane pattern is distinct relative to other commercially popular edible oils. To investigate this further, the analysis of a number of authentic extra virgin and refined olive oils was carried out to determine whether the n-alkane patterns and concentrations could be used as oil identifiers and therefore whether adulteration of olive oil could be detected using this method. In a previous study it was possible to distinguish selected edible oils (soyabean, corn, sunflower, rapeseed) on the basis of their carbon number profiles (Fig. 1).8,9 The n-alkane data for the olive oils, obtained during this study, were compared with equivalent data held on the database, which included rapeseed, safflower, sunflower, palm, palm kernel, coconut, groundnut, corn and soyabean oils.
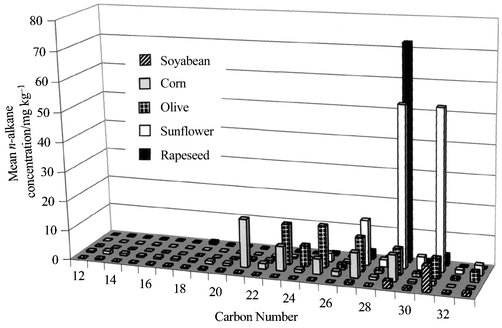 | ||
| Fig. 1 Mean concentration (mg kg−1) for the n-alkanes from various crude edible oils. The variations in the carbon number profiles are clearly observed. Rapeseed oil, for example, comprises mainly nC29 whereas the n-alkanes from sunflower oil are dominated by nC27, nC29 and nC31. The dominant n-alkane in corn oil is nC21 and the concentrations of nC31 and nC33 were relatively minor, whereas the dominant n-alkane in soyabean oil was nC31. In contrast, olive oil was characterised by significant concentrations of odd-numbered n-alkanes from nC23 to nC33. | ||
Experimental
Reagents
Analytical reagent grade isohexane, dichloromethane, methanol, acetone and water were supplied by Rathburn Chemicals (Walkerburn, UK). Squalane (Sq) was obtained from Kodak Eastman Fine Chemicals (New York, USA). The internal standard 2,2,4,4,6,8,8-heptamethylnonane (HMN) was obtained from Aldrich Chemical (Gillingham, Dorset, UK), as were undecane (nC11), dodecane (nC12), tridecane (nC13), pentadecane (nC15), heptadecane (nC17), 2,6,10,14-tetramethylpentadecane (pristane), heneicosane (nC21), docosane (nC22), pentacosane (nC25), heptacosane (nC27), nonacosane (nC29), triacontane (nC30) and tritriacontane (nC33). Tetradecane (nC14), hexadecane (nC16), octadecane (nC18), eicosane (nC20), tricosane (nC23), tetracosane (nC24), hexacosane (nC26), octacosane (nC28) and dotriacontane (nC32) were purchased from Eastman Chemical (New York, USA). Hentriacontane (nC31) and 2,6,10,14-tetramethylhexadecane (phytane) were obtained Restek (Bellefonte, PA, USA). Silicic acid (500 g 100 mesh) was obtained from Promochem (Welwyn, Hertfordshire, UK) and treated as described below.Edible oils
Authentic extra virgin olive oils were obtained through the International Olive Oil Council (Madrid, Spain). Authentic refined olive oils were supplied by the International Olive Oil Council, the Institute de la Grassa (Seville, Spain) and Victor Guedes, Indústria e Comércio (Lisbon, Portugal). Commercial olive oils were purchased from a number of local retail outlets.Samples of olive oil from retail outlets were stored in their retail packaging in the dark at room temperature. Samples of authentic extra virgin olive oil and authentic refined olive oil were stored in the glass bottles in which they were received in the dark at room temperature (20 ± 2 °C).
Preventative measures for reducing background contamination
Hydrocarbons are ubiquitous to the environment and great care must be taken to avoid adventitious contamination of samples. Therefore, all analytical glassware was soaked in Decon 90 (Decon Laboratories, Hove, Sussex, UK) before being thoroughly scrubbed and then rinsed with water. The glassware was then dried in an oven at 85 °C. After cooling, and just prior to use, the glassware was rinsed with dichloromethane and then with isohexane, the latter being allowed to evaporate before proceeding. The columns used for the silicic acid column chromatography were soaked at 3 monthly intervals in concentrated nitric acid to clean the frits. The columns were then washed with copious amounts of water before being washed as described previously.Individual batches of all solvents were checked for n-alkanes by removal of an aliquot (100 ml) to which was added a two-component internal standard (100 μl), containing HMN and Sq in isohexane. This mixture was then concentrated, by rotary evaporation, to ≡300 μl. The solvent was transferred, with washings, to a tapered vial, where it was further concentrated to 25 μl under a stream of scrubbed nitrogen. An aliquot (1 μl) was analysed by gas chromatography with flame ionisation detection (GC-FID).
Preparation of silicic acid
Silicic acid (100 mesh), was heated at 500 °C for 18 ± 2 h. The silicic acid was then cooled prior to deactivation with HPLC grade water (1% w/v).Isolation of n-alkanes
The edible oil (500 mg) was accurately weighed and the internal standard added (200 μl containing 2000–3000 ng each of squalane and heptamethylnonane in isohexane). In the case of refined olive oil, the quantity of internal standard was doubled. To the oil containing internal standard was added isohexane (5 ml). The isohexane–oil solution was applied to a silicic acid column (11 × 2 cm id) which had been pre-washed with isohexane (100 ml). The analytes were then eluted with isohexane (130 ml), the column eluent being collected in a 250 ml round-bottomed flask. The solution was concentrated by rotary evaporation to ≡1 ml and then further concentrated to 300 μl under a stream of scrubbed nitrogen, following transfer to a 1 /2 dram (1.75 ml) vial. The n-alkanes were isolated from any aromatic components by HPLC using a previously calibrated LiChrosorb Si-60, 5 μm column (25 × 0.46 cm id) with a flow rate of 2 ml min−1 of isohexane. The aliphatic fraction was collected over the first 2.5–3 min; the time was dependent on the column’s predetermined split of the aliphatic hydrocarbons from the aromatic hydrocarbons. The aliphatic fraction was then concentrated, using rotary evaporation, to ≡500 μl, then transferred with washings to a tapered vial insert where it was further concentrated, under a stream of scrubbed nitrogen, to 25 μl (75 μl for refined olive oils). An aliquot (1 μl) was analysed by GC-FID. The extraction and analyses were each performed in duplicate with a procedural blank being undertaken with each batch of six duplicate analyses.Preparation of adulterated extra virgin olive oil
Authentic extra virgin olive oil was accurately weighed into a conical flask and to this was added an accurately weighed amount of either crude sunflower or crude rapeseed oil, resulting in adulteration levels of between 0.5 and 11% w/w. The prepared mixtures were ultrasonicated for 15 min to ensure thorough mixing of the oils. Aliquots (500 mg) were accurately weighed and extracted as detailed above. The extraction and analyses were each performed in triplicate with a procedural blank being undertaken with each batch of 12 analyses.Analysis by GC-FID
n-Alkanes were analysed by GC-FID using an HP 5890 Series II gas chromatograph (Hewlett-Packard, Bracknell, Berkshire, UK) fitted with an HP 7673 automatic injector and flame ionisation detector (300 °C) using a fused silica capilliary column (25 cm × 0.25 mm id) coated with a 0.33 μm film of Ulta 1 (Hewlett-Packard). On-column injections were made at 60 °C and after 3 min the temperature was elevated at 4 °C min−1 to 280 °C and held at this temperature until the end of the run. Nitrogen (17 lb in−2) was used as the carrier gas. The data were collected via a PE Nelson 610 link box and processed using Perkin-Elmer Turbochrom version 3.3 software (Perkin-Elmer, Beaconsfield, Bucks., UK). Quantification of the n-alkanes in refined olive oil was complicated by the presence of steriodial hydrocarbons and squalene. Under these circumstances, the oven temperature profile was altered such that after the initial hold of 3 min the temperature was elevated at 25 °C min−1 to 240 °C and then at 0.5 °C min−1 to 252 °C prior to final elevation at 25 °C min−1 to 280 °C where it was held for 20 min.Results and discussion
Quality control
Hydrocarbons are ubiquitous in the environment and therefore particular care has to be taken when carrying out trace analyses for n-alkanes in food matrices to avoid background contamination of sample isolates. Quality control, through regular analysis of a procedural blank, is therefore essential. Furthermore, each sample was analysed in duplicate to ensure that any spurious peaks could be identified. Similarly, it was important that the peak identification was assessed. This was achieved by regularly running a standard mixture containing the internal standards, pristane, phytane and the n-alkanes undecane to tritriacontane. Where appropriate, peak identification was confirmed by GC-MS.Calibration curves were prepared for the n-alkanes nC11–nC33 and for HMN, pristane, phytane and squalane using standard solutions covering approximately the concentration range 1 ng μl−1–100 ng μl−1. Quantification was on the basis of the added internal standard, squalane, since this compound had a retention time approximating the major determinants. The detector response for each of the n-alkanes, pristane, phytane, heptamethylnonane and squalane was linear; correlation coefficients of 0.9991, 0.9998, 0.9998, 0.9997 and 0.9998 were obtained for nC11, nC16, nC23, squalane and nC31, respectively. To ensure that the detector response was maintained a standard mixture containing, nC12, nC25, nC20, nC33, pristane, heptamethylnonane and squalane was analysed at the beginning of each batch of six samples and the resulting data were monitored using Shewhart charts.
The limit of detection of n-alkanes with FID, when using automated integration, was 25 pg on-column. However, it was possible, by manual integration of the peaks, to determine n-alkane levels as low as 5 pg on-column, with a signal-to-noise ratio of 7∶1 in the region of octadecane (nC18). The limit of detection for the procedure was determined to be 2 ng g−1 with the term ‘trace’ being used for concentrations between 2 and 16 ng g−1. These figures were determined by taking a standard nC11–nC33 mixture, containing ≡2 ng of each component, through the analytical procedure, resulting in an on-column concentration equivalent to ≡50 pg.
The recoveries of the n-alkanes were determined by extracting a known amount of a standard mixture, containing tridecame (nC13), heneicosane (nC21), nonacosane (nC29) and squalane, using the same procedure as for edible oils, on six separate occasions. Mean recoveries of at least 86% (s 11.2%) were obtained for nC13, this figure rising to >100% for the remaining components.
n-Alkane concentration and composition of olive oils
Twenty authentic extra virgin olive oil samples, of various origins, were analysed. The n-alkane concentration (nC15–nC33) ranged from 18.6 mg kg−1 in a Spanish olive oil to 175.7 mg kg−1 in a Greek olive oil (Table 1). The mean n-alkane concentration (nC15–nC33) was 71.6 mg kg−1 (n = 20, s = 49.0 mg kg−1; RSD = 68.4%). The n-alkane profile of the extra virgin olive oils was relatively unique. As with all edible oils, the extra virgin olive oil exhibited a distinct odd carbon predominance. Olive oil was, however, characterised by significant concentrations of odd-numbered n-alkanes from nC23 to nC33 (Fig. 2), which contrasts with the reduced principal n-alkane range typical of rapeseed, sunflower or soyabean oils (Fig. 1). The wider range of major n-alkanes observed for the extra virgin olive oil was also noted for corn oil (Fig. 1) but with this oil the principal n-alkane was nC21. The authentic extra virgin olive oil carbon number profiles were also unique because the n-alkane concentrations and distributions were dependent on the origin and variety of the olive (Fig. 3). The highest total n-alkane concentrations (nC15–nC33) were typically observed in the extra virgin olive oils of Greek origin, with one exception. The total n-alkane concentration (nC15–nC33) of this particular oil was 40.8 mg kg−1 compared with a mean total n-alkane concentration of 152.5 mg kg−1 in the other three Greek extra virgin olive oils analysed. The carbon number profile of the Greek extra virgin olive oils was typically characterised by large nC23 and nC25 followed by a steady decrease in the predominance of the odd numbered carbons [Fig. 3(B)]. The total n-alkane concentrations (nC15–nC33) of the Italian extra virgin olive oils ranged from 31.3 to 105.5 mg kg−1 (n = 6, mean = 71.7 mg kg−1, s = 27.7 mg kg−1, RSD = 38.6%). The carbon number profiles of the Italian extra virgin olive oils [Fig. 3(A)] could not be characterised in the same way as those of the Greek samples. The lowest total n-alkane concentrations (nC15–nC33) were observed in the extra virgin olive oils of Spanish origin, ranging from 18.6 to 69.5 mg kg−1 (n = 6, mean = 43.6 mg kg−1, s = 19.6 mg kg−1, RSD = 45.0%). Similarly to the Italian extra virgin oils, the carbon number profiles of the Spanish oils were not characterised by a single profile [Fig. 3(C) and (D)].| Mean n-alkane concentration of duplicate analyses/mg kg−1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | nC25 | nC26 | nC27 | nC28 | nC29 | nC30 | nC31 | nC32 | nC33 | Sum nC15–nC33 |
| EXO01 | 9.6 | 1.4 | 15.8 | 1.2 | 9.3 | 0.8 | 5.5 | 0.4 | 2.46 | 56.3 |
| EXO02 | 9.4 | 0.9 | 3.4 | 0.4 | 4.5 | 0.5 | 4.8 | 0.6 | 3.2 | 40.8 |
| EXO03 | 37.4 | 3.3 | 21.6 | 2.0 | 12.4 | 1.6 | 7.3 | 0.9 | 3.6 | 175.7 |
| EXO04 | 20.1 | 2.1 | 14.0 | 1.3 | 11.2 | 1.4 | 9.7 | 1.1 | 5.0 | 105.5 |
| EXO05 | 6.8 | 1.1 | 8.1 | 1.1 | 9.9 | 1.4 | 11.0 | 1.1 | 6.0 | 53.7 |
| EXO06 | 8.6 | 1.2 | 11.6 | 0.8 | 9.0 | 0.8 | 6.7 | 0.6 | 3.3 | 49.7 |
| EXO07 | 20.9 | 2.0 | 11.4 | 1.1 | 9.8 | 1.0 | 8.7 | 0.9 | 4.7 | 93.0 |
| EXO08 | 30.9 | 2.7 | 19.2 | 1.8 | 11.9 | 1.5 | 8.3 | 1.0 | 4.3 | 151.1 |
| EXO09 | 9.1 | 1.5 | 16.8 | 0.3 | 10.5 | 1.1 | 7.4 | 0.7 | 3.7 | 58.5 |
| EXO10 | 3.2 | 0.9 | 4.7 | 0.8 | 4.9 | 0.7 | 3.4 | 0.4 | 2.0 | 23.4 |
| EXO11 | 11.9 | 1.6 | 11.5 | 1.4 | 14.0 | 1.7 | 15.7 | 1.8 | 8.5 | 85.9 |
| EXO12 | 28.0 | 2.5 | 15.8 | 1.5 | 10.0 | 1.2 | 6.9 | 0.8 | 3.5 | 130.6 |
| EXO13 | 6.6 | 0.8 | 4.6 | 0.2 | 5.0 | 0.5 | 2.6 | 0.3 | 1.1 | 31.3 |
| EXO14 | 9.5 | 1.3 | 9.8 | 0.2 | 8.1 | 1.1 | 6.3 | 0.7 | 3.1 | 54.5 |
| EXO15 | 18.8 | 4.3 | 16.3 | 0.9 | 9.5 | 0.9 | 5.4 | 0.6 | 2.9 | 66.9 |
| EXO16 | 9.2 | 1.2 | 9.1 | 0.9 | 6.2 | 0.7 | 3.4 | 0.4 | 1.4 | 42.1 |
| EXO17 | 2.2 | 0.7 | 3.3 | 0.7 | 3.8 | 0.6 | 3.5 | 0.4 | 1.7 | 18.6 |
| EXO18 | 11.3 | 1.9 | 9.7 | 1.4 | 8.7 | 1.3 | 6.4 | 0.7 | 2.4 | 58.5 |
| EXO19 | 12.1 | 1.9 | 16.3 | 1.5 | 11.9 | 1.2 | 7.0 | 0.6 | 2.9 | 69.5 |
| EXO20 | 16.7 | 1.6 | 3.8 | 0.5 | 4.7 | 0.6 | 4.8 | 0.5 | 2.9 | 64.6 |
| Mean | 14.1 | 1.7 | 11.3 | 1.0 | 8.8 | 1.0 | 6.7 | 0.7 | 3.4 | 71.6 |
| s | 9.3 | 0.9 | 5.6 | 0.5 | 3.0 | 0.4 | 3.0 | 0.3 | 1.7 | 49.0 |
| RSD (%) | 66.2 | 51.6 | 49.1 | 52.2 | 34.2 | 36.8 | 45.0 | 46.4 | 50.0 | 68.4 |
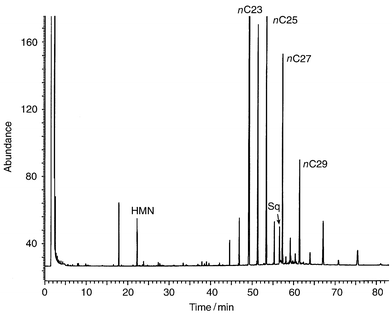 | ||
| Fig. 2 Gas chromatogram of a typical extra virgin olive oil. The profile shows an odd carbon predominance from nC23. The internal standards 2,2,4,4,6,8,8-heptamethylnonane (HMN) and squalane (Sq) are clearly evident. | ||
 | ||
| Fig. 3 Carbon number profiles for four of the 20 authentic extra virgin olive oils analysed. (A) Italian extra virgin olive oil; (B) Greek extra virgin olive oil; (C) and (D) profiles of two Spanish extra virgin olive oils. | ||
Twenty samples of authentic refined olive oils were analysed, the total n-alkane concentrations (nC15–nC33) ranging from 15.0 mg kg−1 in a Spanish olive oil to 174.3 mg kg−1 in a Tunisian olive oil. There was limited availability of authentic samples of refined olive oil and therefore the majority of the oils analysed were of Spanish origin. The mean n-alkane concentration (nC15–nC33) was 36.2 mg kg−1 (n = 20 s = 36.92 mg kg−1, RSD = 102.0%). Analysis of the authentic refined olive oils was complicated by the presence of sterenes and squalene.16 During the refining process squalene and its oxidised products undergo chemical transformations including cyclisation, isomerisation and dehydration, yielding a great number of hydrocarbons.18,19 These compounds interfered such that, under the standard GC oven parameters, the n-alkanes were not resolved owing to a UCM (unresolved complex mixture) in the region of nC28. The majority of n-alkanes were resolved by altering the oven temperature profile such after after an initial delay of 3 min the oven temperature was elevated at 25 °C min−1 to 240 °C and then at 0.5 °C min−1 to 252 °C prior to final elevation at 25 °C min−1 to 280 °C, where it was maintained for 20 min. The overwhelming presence of squalene in several of the samples however, had the effect of masking the nC28 peak.
The effect of the refining of the olive oil was typically to reduce the concentration of the shorter chain length n-alkanes. As with the extra virgin olive oils, the odd numbered predominance was not limited to one or two n-alkanes but was spread over several n-alkanes from nC23 onwards, with the exception of one sample, where the odd numbered predominance was from nC21 onwards.
Twenty retail samples of olive oil were purchased from local outlets. Of these, 10 were described as extra virgin olive oils with the remainder being described as olive oils. The total n-alkane concentration (nC15–nC33) of the extra virgin olive oils ranged from 25.7 mg kg−1 in an oil of Spanish origin to 164.3 mg kg−1 in a Greek oil, with a mean of 79.0 mg kg−1 (n = 10, s = 49.9 mg kg−1, RSD = 63.1%). Similarly to the authentic extra virgin olive oils, the highest total n-alkane concentrations were observed in the two retail samples of Greek origin. The carbon number profiles were also typical of the Greek extra virgin olive oils with characteristic large nC23 and nC25 followed by a steady decrease in the predominance of the odd numbered carbons. Three retail extra virgin olive oils were of Italian origin. The mean total n-alkane concentration (nC15–nC33) of these oils was 70.8 mg kg−1 (s = 14.9 mg kg−1), which was very similar to the mean of the authentic extra virgin olive oils of Italian origin (71.7 mg kg−1, s = 27.7 mg kg−1). The two retail samples, of Spanish origin, had low total n-alkane concentrations of 25.7 and 51.2 mg kg−1; this was as found for the authentic samples.
The total n-alkane concentration (nC15–nC33) of the retail olive oils ranged from 11.1 to 75.8 mg kg−1 (n = 10, mean = 33.9 mg kg−1, s = 22.0 mg kg−1, RSD = 64.8%); this figure was very similar to that observed for the authentic refined olive oils. These figures may not be truly reflective, however, inasmuch as the majority of the authentic samples were of Spanish origin, and the origin of all but two of the retail samples was unknown. Typical of oils which have been refined, there was a reduction in the concentrations of the shorter chain length n-alkanes and the introduction of steroidal hydrocarbons. Similarly to the authentic refined olive oil, a small, but distinct, UCM in the region of nC28 and squalene was observed in nine of the retail olive oils. No UCM was observed in one sample of retail olive oil, although there was evidence of refining due to the presence of sterenes.
Statistical significance of the n-alkane concentrations and profiles
A previous study found that pattern recognition of edible oils was possible through principal component analysis (PCA).8 Initially PCA was done solely on the olive oils based on the nC15–nC33 distribution, because the n-alkane concentration and composition appeared to indicate that they were region specific. Extra virgin olive oils could not be distinguished by a single carbon number profile. By plotting the first principal component against its second, however, it was found that specific data clusters were obtained which clearly differentiated the olive oils of Greek origin from those of the olives of Spanish and Italian origin (Fig. 4). PCA analysis did not allow for the differentiation of extra virgin olive oil from that of refined olive oil. | ||
| Fig. 4 Results of PCA of the n-alkanes (nC15–nC33) in authentic extra virgin olive oils (○) and commercial extra virgin olive oils (□). By plotting the first principal component against the second principal component the Greek oil (G) is well resolved from the Italian (I) and Spanish (S) oils. | ||
In the previous study,8,9 it was found that oil specific clusters were obtained which clearly differentiated sunflower, rapeseed and corn oil. The other oils (soyabean, palm, palm kernel, coconut and groundnut) formed a fourth cluster. Data for safflower and groundnut oils, analysed as part of a separate project, could also clearly be differentiated from the other vegetable oils (Fig. 5).
 | ||
| Fig. 5 Results of PCA of the n-alkanes (nC25–nC33) in crude edible oils obtained by plotting the first principal component against the second principal component. The extra virgin olive oil is clearly resolved from the other edible oils. Safflower oil, sunflower oil, corn oil and rapeseed oil can also be clearly differentiated. | ||
The data for the extra virgin olive oils were incorporated into the database. The principal components were investigated for nC11–nC33, nC15–nC33, nC23–nC33 and nC25–nC33. The first principal only accounted for ≡40% of the variation using the data for nC15–nC33; however, this figure increased to ≡70% of the variation if the data for nC25–nC33 were used. The PCA was therefore based on the data for nC25–nC33. By plotting the first principal component against its second it was found that specific clusters were obtained which clearly differentiated the extra virgin olive oils from the other vegetable oils (Fig. 5). By plotting the second principal component against its third a different range of clusters was obtained. This allowed for the clear differentiation of rapeseed, sunflower, soyabean, corn and safflower oils.
Adulteration of an authentic extra virgin olive oil with crude sunflower and crude rapeseed oils
An authentic extra virgin olive oil was adulterated with either crude sunflower oil at levels of 0.5, 1.1, 2.6, 5.4 and 11.2% w/w or crude rapeseed oil at levels of 0.5, 1.3, 2.6, 5.3 and 11.3% w/w. The crude sunflower and crude rapeseed oils were analysed so as to determine their n-alkane concentrations (nC15–nC33) and carbon number profiles. The carbon number profiles of the two oils were found to be the same as those of the crude rapeseed and sunflower oils analysed in the previous study.8,9 A single n-alkane dominated the hydrocarbons from crude rapeseed oil, that of nC29 with a concentration of 108.9 mg kg−1 from a total n-alkane concentration (nC15–nC33), for this oil, of 122.6 mg kg−1 [Fig. 6(A)]. Two n-alkanes dominated the hydrocarbons from the crude sunflower oil, viz., nC29 and nC31, with concentrations of 58.4 and 68.9 mg kg−1, respectively, from a total n-alkane concentration (nC15–nC33) of 162.7 mg kg−1 [Fig. 6(A)]. The concentrations of the dominant n-alkanes in the crude sunflower and rapeseed oils were considerably greater than those of their corresponding n-alkanes in the extra virgin olive oil (nC29 4.7 mg kg−1, nC31 4.8 mg kg−1). Hence the carbon number profiles were distinct for each of the oils [Fig. 6(A)]. | ||
| Fig. 6 Carbon number profiles of (A) an authentic extra virgin olive oil, crude sunflower oil and crude rapeseed, (B) authentic extra virgin olive oil adulterated with 0.5, 1.3, 2.6, 5.3 and 11.3% of crude rapeseed oil and (C) authentic extra virgin olive oil adulterated with 0.5, 1.1, 2.6, 5.4 and 11.2% of crude sunflower oil. | ||
Despite the adulteration of the extra virgin olive oil with various concentrations of crude rapeseed oil, there was no significant effect on the total n-alkane concentration. The adulteration with crude rapeseed oil did, however, result in an increase in the relative concentration of nC29 [Fig. 6(B)]. This increase was particularly noticeable in the 11, 5 and 2.5% mixtures where the nC29 concentrations were 15.3, 9.7 and 7.3 mg kg−1, respectively, relative to 4.7 mg kg−1 in the original olive oil. This resulted in very distinct n-alkane profiles for the samples when the concentration was plotted against carbon number. Using the carbon number profiles alone it was possible to determine adulteration of the extra virgin olive oil with as little as 2.5% crude rapeseed oil.
The adulteration of extra virgin olive oil with crude sunflower oil resulted in a slight increase in the total n-alkane concentration of only the 11% mixture [Fig. 6(C)]. Similarly to the samples adulterated with the crude rapeseed oil, it was the variation in the individual n-alkane concentrations which was particularly noticeable. The concentrations of both nC29 and nC31 were greater in the samples with 2.5, 5 and 11% crude sunflower oil present. This again resulted in very distinct n-alkane profiles for the samples when the concentration was plotted against carbon number (Fig. 6). Using the carbon number profiles alone it was possible to distinguish as little as 2.5% crude sunflower oil in the olive oil.
Statistical significance of the n-alkane concentrations and profiles of the adulterated oils
The use of carbon number profiles alone allowed the determination of adulteration of extra virgin olive oil with as little as 2.5% of rapeseed or sunflower oil. The use of PCA, however, allowed the determination of adulteration with as little 0.5% of adulterant. By plotting the first principal component against its second, the authentic extra virgin olive oil was clearly differentiated from all the samples which had been adulterated (Fig. 7). Adulteration led to the depression of the spikes at nC23 and nC25 associated with this olive oil and lowered the values associated with nC21 and nC26 generally. The extra virgin olive oil chosen for the adulteration experiments was statistically found to be typical of extra virgin olive oil and therefore this method could be used in conjunction with carbon number profile to authenticate an olive oil. | ||
| Fig. 7 Results of PCA of the n-alkanes (nC21–nC33) in extra virgin olive oil adulterated with either rapeseed or sunflower oil obtained by plotting the first principal component against the second principal component. The extra virgin olive oil is clearly resolved from the adulterated samples. 1 and 2, ≡11% adulteration; 3 and 4, ≡5% adulteration; 5 and 6, ≡2.5% adulteration; 7 and 8, ≡1% adulteration; and 9 and 10, ≡0.5% adulteration. Even numbers, adulterant was rapeseed oil; odd numbers, adulterant was sunflower oil. | ||
References
- D. E. Lester, Prog. Food Nutr. Sci., 1979, 3, 1 Search PubMed.
- A. P. Tulloch, in Chemistry and Biochemistry of Natural Waxes, ed. P. E. Kolattukudy, Elsevier, Amsterdam, vol. 241, 1976, pp. 235–287. Search PubMed.
- E. Fedeli and G. Jacini, Adv. Lipid Res., 1971, 9, 335 Search PubMed.
- M. Bastic, L. J. Bastic, J. A. Jovanovic and G. Spiteller, J. Am. Oil Chem. Soc., 1978, 55, 886 Search PubMed.
- P. E. Kolattukudy, J. S. Buckner and L. Brown, Biochem. Biophys. Res. Commun., 1972, 47, 1306 CrossRef CAS.
- A. Khan and P. E. Kolattukudy, Biochem. Biophys. Res. Commun., 1974, 61, 1379 CAS.
- A. S. McGill, C. F. Moffat, P. R. Mackie and P. Cruickshank, J. Sci. Food Agric., 1993, 61, 357 CAS.
- C. F. Moffat, P. Cruickshank, N. A. Brown, D. Mennie, D. A. Anderson and A. S. McGill, The Concentration and Composition of n-Alkanes in Edible Oils, Report submitted to Food Science Division, MAFF, Ministry of Agriculture Fisheries and Food, London, 1995. Search PubMed.
- P. Cruikshank, D. A. Anderson and C. F. Moffat, in Proceedings of the International Conference on Plant Oils and Marine Lipids, ed. C. J. O’Connor, New Zealand Institute of Chemistry, 1997, p. 178. Search PubMed.
- A. Dolamore, A Buyer’s Guide To Olive Oil, Grub Street, London, 1994, pp. 6–8. Search PubMed.
- D. Firestone, J. L. Summers, R. J. Reina and W. S. Adams, J. Am. Oil Chem. Soc., 1985, 62, 1558 Search PubMed.
- D. Firestone, K. L. Carson and R. J. Reina, J. Am. Oil Chem. Soc., 1988, 65, 788 Search PubMed.
- Codex Alimentarius, EC Commission of the Codex Alimentarius, 1993, CL 1993/15-FO..
- Off. J. Eur. Commun., Regulation No. 2568/91, 1991, No. L248..
- R. Aparicio, M. T. Morales and V. J. Alonso, J. Agric. Food Chem., 1997, 45, 1076 CrossRef CAS.
- J. P. Favier, D. Bicanic, J. Cozijnsen, B. van Veldhuizen and P. J. Helander, J. Am. Oil Chem. Soc., 1998, 75, 359 Search PubMed.
- I. J. Wesley, F. Pacheco and A. E. J. McGill, J. Am. Oil Chem. Soc., 1996, 73, 515 Search PubMed.
- D. Mennie, C. F. Moffat and A. S. McGill, J. High Resolut. Chromatogr., 1994, 17, 831 CAS.
- A. Lanzon, T. Albi, A. Cert and J. Gracian, J. Am. Oil Chem. Soc., 1994, 71, 285 Search PubMed.
Footnote |
| † Presented at SAC 99, Dublin, Ireland, July 25–30, 1999. |
| This journal is © The Royal Society of Chemistry 2000 |
