Determination of colour developing agents in photographic processing solutions using fixed-volume single-use sensors†
John W. Dilleen*a, Gowri V. Depa, Brian J. Bircha, Barry G. D. Haggetta and Steve J. Edwardsb
aSensors and Cryobiology Research Group, Research Centre, The Spires, 2 Adelaide Street, Luton, UK LU1 5DU. E-mail: john.dilleen@luton.ac.uk;; Web: http://www.luton.ac.uk/scrg
bKodak European Research, Headstone Drive, Harrow, Middlesex, UK HA1 4TY
First published on UnassignedUnassigned7th January 2000
Abstract
An electrochemical method for the determination of colour developing agents in photographic processing solutions using single-use sensors is described. The sensor consists of two parts: the transducer, based on a screen-printed carbon (working and reference) and gold (counter) three-electrode configuration; and a sampler/electrochemical cell. The parts fit together to give a single-shot sampling and measurement device. The sensors were used to monitor the concentrations of the colour developing agent CD-4 (i.e. 4-(N-ethyl-N-2-hydroxyethyl)-2- methylphenylenediamine sulfate) in a photoprocessor. Comparison with HPLC data showed that there was a good correlation between the two methods (R2 = 0.97). The sensor method was more convenient to use than HPLC and enabled measurements to be carried out at the photoprocessor without need to send samples to a laboratory for analysis.
Introduction
In many situations, single-use sensors have a number of actual or potential benefits. In the context of this paper, such devices have the following attributes: the sensors do not require calibration since they are calibrated in manufacture; the sensors do not require any maintenance since they are used for just one measurement; the sensors are unaffected by previous samples since a new sensor is used each time a determination is made.Given the advantages of these measurement devices, it is not surprising that they find applications outside of the laboratory and in the hands of other than trained analysts. Single-use sensor systems have been already realised for a number of applications in clinical diagnostics1,2 and environmental monitoring.1 The following paper describes an electrochemical sensor system for application within a production environment where it would otherwise be normal for samples to be removed to a remote laboratory for analysis.
To obtain satisfactory and reproducible results, it is necessary to maintain within close control limits the processing steps of any photographic operation, particularly where colour photography is concerned. Conventionally, such controls are based essentially on sensitometric techniques.3 Reference charts are constructed for the process control operation based on typical systematic variations of chemical solution and physical parameters. An important parameter in the control process is the concentration of colour developing agent.
Colour developer agent
The chemical compound in colour developing solutions that reduces silver ions to silver is termed the colour developing agent. One such class of organic agents includes p-phenylenediamine (PPD) and its derivatives. CD-4 is a common example used, for example, in the Kodak Flexicolor™ C-41 process.In general PPD undergoes a two-electron oxidation to quinonediimine (QDI) via the semiquinone (SH+) (eqn. (I) and (II)).3 For colour coupling the required form of QDI has a + 1
 | (I) |
 | (II) |
The measurement of CD-4 based on the electrochemical oxidation of PPD at the carbon working electrode of a single-use sensor is described in this paper.
| 2SH+ ⇆ PPD + QDI+ + H+ | (III) |
Experimental
Equipment and reagents
Voltammetric experiments were performed using an electrochemical workstation (Autolab PGSTAT10, Eco Chemie B.V., Utrecht, The Netherlands) with PC software control (GPES 4.3, Eco Chemie B.V.) or a hand-held measurement system, Chemilox™ (Oxley Developments Company Ltd, Ulverston, UK).A three-electrode cell was used, consisting of a screen-printed carbon working electrode, a carbon pseudo-reference electrode and a gold counter electrode. CD-4 was obtained from Kodak and deionised water was from a reverse osmosis water purification system. Flexicolor™ was the colour developer used (Kodak Catalogue No: 368 6250—to make 3.8 l). It consists of three parts (aqueous solutions):4 Part A, potassium carbonate, diethylenetriaminepentaacetic acid, pentasodium salt, sodium bisulfite, potassium bicarbonate; Part B, hydroxylamine sulfate, and Part C, CD-4, sodium bisulfite.
Solutions were prepared using Parts A and B with sodium bisulfite and known amounts of CD-4 added separately.
Sensor fabrication
Commercial prototype sensors were fabricated (Oxley Developments Company Ltd) by screen-printing, using suitable inks, onto an alumina substrate. The printed substrate measured 1 cm × 9 cm, with gold connectors and connecting tracks printed as part of each of the three electrodes (Fig. 1). The electrochemical cell was formed by clipping an injection moulded top-cap over the alumina substrate on top of the electrodes so as to give a gap (ca. 0.75 mm) between the inside of the top-cap and the electrode surfaces. The electrodes completed electrical connection to the potentiostat via a three way sprung-pin connector. | ||
| Fig. 1 Schematic layout of the sensor configuration. The electrodes were hidden behind the top-cap that formed the measurement cell. | ||
Sampling and measurement
The sensors were immersed vertically in the developer solution to a level just above the top-cap and inserted into the connector. The total sample volume was approximately 150 μl, and was held in place by surface-tension and the physical structure of the top-cap.In our laboratory, voltammetric measurements were made using linear scan voltammetry from 0 V to 0.65 V vs. a carbon pseudo-reference electrode at 20 mV s−1 using the Autolab potentiostat. Cyclic voltammetry in the same potential range was used for qualitative studies of the electrode processes.
For evaluation with real photoprocessing solutions, measurements were made using the Chemilox™ instrument.
Results and discussion
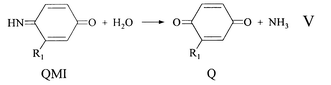
The cyclic voltammetric curve (CV) characterising CD-4 oxidation in Flexicolor™ developer at pH 10.2 is shown in Fig. 2. With respect to the forward scan the anodic peaks at 0.26 V and 0.43 V represent two sequential oxidation steps. The electrochemistry of PPD is difficult to interpret in developer solutions. The product of oxidation, QDI+, is unstable in aqueous solution, undergoing homogeneous hydrolysis first to the quinonemonoimine (QMI), eqn. (IV), then to the quinone (Q), eqn. (V).3,5 Also, QDI+ can undergo addition reactions with nucleophiles, e.g. PPD, sulfite, and coupler ions which may be present in the seasoned developer. Formation of azo dyes can also occur.3 | (IV) |
 | (V) |
![Cyclic voltammetric curve for CD-4 in Flexicolor™ developer.
[CD-4] = 4 g l−1. Scan rate: 20 mV
s−1.](/image/article/2000/AN/a906845g/a906845g-f2.gif) | ||
| Fig. 2 Cyclic voltammetric curve for CD-4 in Flexicolor™ developer. [CD-4] = 4 g l−1. Scan rate: 20 mV s−1. | ||
In model developer solutions, the anodic oxidation peaks are closely correlated to the concentration of CD-4 (4–6 g l−1), Fig. 3. In seasoned developer, the first peak data gave the best correlation with HPLC analysis of PPD. The second peak may be due to oxidation of species formed as a result of the first oxidation. Sulfonation of QDI and QMI is known to occur in developer solutions.3 Homogeneous side reactions may account for the irreversible nature of the CV.
 | ||
| Fig. 3 Calibration data best linear fit for CD-4 using linear scan peak height data; absolute peak height current with zero current baseline. (A) First peak, y = 20.2x + 18.8, R2 = 0.991. (B) Second peak, y = 40.8x + 1.27, R2 = 0.994, n = 3. 3ς error bars. | ||
The first oxidation peak was used to measure the reduced form of CD-4 (PPD) in photoprocessors. The Chemilox™ measurement system was used to make measurements of CD-4 in the developer tank of a photoprocessing unit over a 19 day period. The results were compared to quantitative HPLC analysis of CD-4 in samples taken at the same time. Fig. 4A shows a plot of Chemilox™ against HPLC for the analysis of CD-4 in a photoprocessing unit (R2 = 0.97). The equation for the line of best fit was used to calculate CD-4 concentration using Chemilox™, Fig. 4B. With this approach the correlation coefficient, R, between CD-4 concentration determined by HPLC and by Chemilox™ was 0.98.
 | ||
| Fig. 4 Plots of Chemilox™ against HPLC for the analysis of CD-4 in a photoprocessing unit. | ||
Conclusions
It was demonstrated that single-use sensors could be used to measure the colour developing agent CD-4 in photoprocessing solutions with both accuracy and precision. The sensors were much more convenient to use than HPLC or sensitometry and enabled measurements to be made at the processor within a few minutes rather than having to send samples to a laboratory for analysis.The sensors can be similarly used for other colour developing agents, and reagents may be incorporated into the top-caps to enable measurement of a range of analytes in environmental and industrial samples (e.g. silver in photoprocessing solutions and in effluent6).
Acknowledgements
Grateful thanks are extended to Kodak Limited for funding this work, and to Oxley Developments Company Limited (particularly Dr Andrew Bell) who provided the commercial prototype sensors and instrumentation.References
- J. P. Hart and S. A. Wring, Tr. Anal. Chem., 1997, 16, 89 Search PubMed.
- C. B. Wilson, Br. Med. J., 1999, 319, 1288 Search PubMed.
- T. H. James, The theory of the photographic process, Macmillan Publishing Co., Inc., 1977, 4th edn. Search PubMed.
- Kodak internet publication; http://www.kodak.com/US/en/nav/support.shtml. Material Safety Data Sheets for Cat. No. 368 6250..
- TH. Wandlowski, D. Gosser, Jr., E. Akinele, R. De Levie and V. Horak, Talanta, 1993, 40, 1789 CrossRef CAS.
- J. W. Dilleen, S. D. Sprules, B. J. Birch and B. G. D. Haggett, Analyst, 1998, 123, 2905 RSC.
Footnote |
| † Presented at SAC 99, Dublin, Ireland, July 25–30, 1999. |
| This journal is © The Royal Society of Chemistry 2000 |
