Investigation of amine and polyol functionality in extracts of polyurethane wound management dressings using MALDI-MS†
Naaman Ostah*a, Graham Lawsona, Shazia Zafara, Glynn Harringtonb and John Hicksb
aDe Montfort University, Department of Chemistry, The Gateway, Leicester, UK LE1 9BH
bWound Management Division, Smith & Nephew Medical Ltd., 101 Hessle Road, Hull, UK
First published on UnassignedUnassigned7th January 2000
Abstract
Polyurethane (PU) foams used in wound management are produced by a reaction between aromatic diisocyanates and polyether polyols. There is concern that residues of these starting materials, which may contain aromatic amine functionality, may leach from the finished polymer during in vivo applications. Furthermore, oligomers and additives may be leached from the PU system after the polymerization process is complete. Finished polymers have, therefore, been extracted with a range of solvents, such as water, diethyl ether and dilute HCl. The extracts were subjected to MALDI-MS (matrix-assisted laser desorption ionization mass spectrometry) analysis in an attempt to determine the amine and polyol functionality. Direct MALDI-MS analysis of the wound dressing extracts indicated the presence of the polyol used in the formulation of the foam. The spacing between the peaks identified the base monomer used in the polyol. MALDI-MS analysis of the fluorescamine derivatives of model amine compounds has demonstrated the anticipated increase in mass (278 for monoamines and 278 and 556 for diamines). Similar results were obtained from the derivatization of model polyols with phenyl isocyanate, where the mass shift (n × 119) was a direct measure of the number of active hydroxyl groups. Fluorescamine labelling of PU foam extracts shows the colour change indicative of the presence of an amine, but the subsequent MALDI-MS analysis was unable to demonstrate the anticipated increase in mass.
Introduction
The general aim of this study was to investigate the nature of the chemicals which may be leached from polyurethane wound management materials using matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS).MALDI-MS is an analytical technique which has been recently developed with the aim of analyzing large biomolecules such as fragments of DNA, proteins and oligosaccharides. Since its development, applications of this technique have been extended to include the analysis of synthetic polymeric systems.1–4 The discovery of matrix systems based on organic solvents has allowed the application of this method to low and medium molecular weight polymers which are not water soluble.5–7 This would make it possible to determine directly the composition of different species contained within each polymer sample as well as different functional groups within the extractable oligomer series.
MALDI-MS is a soft ionization technique which allows desorption and ionization of very large molecules even if in complex mixtures, therefore dispensing with the need for time consuming sample derivatization and prior separation. Because of the nature of the ionization in MALDI-MS, very little or no fragmentation of the molecules takes place inside the mass spectrometer, allowing for the simultaneous molecular weight determination of different polymeric species.
This study is concerned with polyurethane foam, which is used in the manufacture of wound management dressings. The manufacture of typical polyurethane foam involves the following steps:(a) Formation of prepolymer: polyol + diisocyanate = polyurethane prepolymer(b) Foaming reaction: (i) prepolymer + water + blowing agent = polyisocyanate (ii) polymerization via urea linkages = foam
These reactions may not proceed to 100% completion and therefore various oligomeric residues may be left in the foam. These oligomers may, under certain circumstances, leach out from the bulk polyurethane material. It is therefore important to keep the levels of oligomeric materials present in these foams to a minimum and to ensure that any oligomers which are capable of leaching out are non-toxic. In this study, the nature of the oligomeric materials extracted from a number of polyurethane foam samples, used in wound management dressings, was extensively investigated using MALDI-MS. A range of solvents was used in the extraction process (diethyl ether, water and dilute hydrochloric acid) in order to ensure maximum results in terms of material extracted. After the molecular weight range of the extracted material had been established, two derivatization procedures were carried out: (a) Reaction with fluorescamine for amine functionality; this is a well established procedure for detection of functional amine groups.8 (b) Reaction with a monoisocyanate for determination of functional hydroxyl end groups.
MALDI mass spectra of the derivatized products were obtained and conclusions drawn based on the shift in the molecular weight of the components of the model polyol.
Experimental
All MALDI-MS experiments were carried out using a Finnigan Lasermat-2000 instrument.Matrix preparation
2,5-Dihydroxybenzoic acid (DHBA) was used as the matrix for sample plate preparation for all the MALDI-MS experiments.The matrix solution was prepared by dissolving 10 mg of DHBA in 1 ml of de-ionized water. A 1 μl aliquot of this solution was mixed with 1 μl of analyte solution.
Derivatization of polyol with phenyl isocyanate
The polyol (7.0 g) in a flask was placed in a water-bath at 70 °C. After equilibrium had been reached, 0.2% dioctyltin dilaurate catalyst was added. Phenyl isocyanate (2.7 g) was added dropwise to the polyol mixture. The reaction was stopped after 3 h. Finally, ethanol (0.3 g) was added to the resulting mixture to remove any unreacted phenyl isocyanate.Stirring was maintained throughout the experiment which was carried out in an inert atmosphere of nitrogen.
Fluorescamine reactions
Fluorescamine reactions were carried out by mixing 1 ml of 10−3 M fluorescamine solution with 10 ml of each of the following solutions: 10−4 M 4,4′-methylenedianiline (MDA) in water; 10−4 M 2,4-toluenediamine (TDA) in water; 1% solution of the aqueous extract; 1% solution of 0.1 M HCl.Wound dressings extract
Various polyurethane wound dressings manufactured by Smith & Nephew Wound Management were extracted with 0.1 M HCl, de-ionized water and diethyl ether.Approximately 20 g of foam were cut into approximately 1 cm3 pieces and placed in 400 ml of the appropriate solvent and left in the dark for 48 h. The extracts were concentrated to approximately 50 ml and then analyzed by MALDI-MS using DHBA as a matrix for sample plate preparation. In each case the analyte solution was mixed with the matrix in a 1∶1 ratio. The samples were allowed to dry at room temperature.
Investigation of amine functionality
Fluorescamine,9 which is a specific reagent for the detection of primary amine groups, was used in a range of model studies. The addition of each fluorescamine molecule should result in an increase of 278 mass units for each amine group in the original analyte (Scheme 1). In principle, this increase in mass can be detected by MALDI-MS. | ||
| Scheme 1 Reaction of a primary amine with fluorescamine. | ||
Reactions of fluorescamine were investigated using aniline and cyclohexylamine as model mono-substituted compounds and both TDA and MDA as examples of residual amines likely to be found in different polyurethane systems. The anticipated masses of the corresponding species before and after the reaction with fluorescamine are given in Table 1. After confirming the specificity of this reaction for a range of model compounds, the polyurethane extracts were subjected to the same procedure of reaction with fluorescamine followed by MALDI-MS analysis.
| Amine | Mr | Mass + 278 | Mass + 556 |
|---|---|---|---|
| Cyclohexylamine | 99 | 377 | — |
| Aniline | 93 | 371 | — |
| TDA | 122 | 400 | 679 |
| MDA | 198 | 477 | 755 |
Investigation of hydroxyl functionality
Phenyl isocyanate (Mr 119) was used to target selectively the hydroxyl groups which are present in polyether polyols. The increase in mass measured by MALDI-MS after reaction can be used to determine the number of hydroxyl groups (Scheme 2). | ||
| Scheme 2 Reaction of polyols with phenyl isocyanate. | ||
Where there is only one OH group present, a mass shift of 119 would be expected. However, with polyols a shift of X multiplied by 119 is expected, where X = number of OH groups.
Results and discussion
(a) Model fluorescamine and amine reactions
The MALDI-MS results of the model studies using aniline, cyclohexylamine, MDA and TDA all showed the expected increase in mass as detailed in Table 1 (278 for monoamines, 278 and 556 for diamines).Fig. 1 shows the resulting MALDI mass spectrum for the reaction of TDA and fluorescamine (%RA = per cent relative abundance). It can be seen that additional peaks were also present (m/z 382, 643, 661). These are believed to be due to the formation of a lactone ring10 in the fluorescamine molecule as shown in Scheme 3. The formation of such a species results in the loss of 18 mass units, which accounts for peaks at m/z 382 and 661 in the spectrum for TDA. TDA is a difunctional amine and therefore the lactone ring formation can occur twice, resulting in the loss of 36 mass units, which accounts for the peak at m/z 643.
 | ||
| Fig. 1 MALDI mass spectrum of TDA and fluorescamine. | ||
 | ||
| Scheme 3 Lactone formation. | ||
(b) Study of polyol functionality
MALDI mass spectra of polyether polyols are complex due to the presence of a large number of oligomers. For example, polyoxyethylene polyols gave a series of mass spectral peaks differing by 44 mass units, which in this case can be attributed to the –CH2CH2O group. However, the spectrum was complicated further than anticipated due to the presence of cationated (Na+ and K+) ions.9 Sodium ions occur frequently in MALDI mass spectra, particularly when glass apparatus has been used. Both sodium and potassium hydroxide may be used in the manufacture of polyols and could therefore give rise to the observed contamination.The MALDI mass spectrum of the reaction product of polyoxyethylene polyol with phenyl isocyanate showed that the molecular weight distribution of the polyol has shifted by 357 mass units.
The mass shift is expected to be a multiple of 119 (molecular weight of phenyl isocyanate) depending on the OH functionality of the polyol. In this case the mass shift of 357 is consistent with a trifunctional polyol.
(c) Study of wound dressing extracts
The aqueous and acid extracts from polyurethane wound dressings both gave complex MALDI mass spectra, as shown in Fig. 2 and 3, showing at least two families of peaks. Each member of a family was found to differ from its neighbours by 44 mass units. This was attributed to the –CH2CH2O unit, thus revealing that the polyols were based on an oxyethylene polyol. | ||
| Fig. 2 MALDI mass spectrum of aqueous foam extract. | ||
 | ||
| Fig. 3 MALDI mass spectrum of acid foam extract. | ||
Diethyl ether extracts of the wound dressings gave no MALDI mass spectra.
It is likely that the presence of different families of peaks is due to the association of the polymer molecules with different metal ions (e.g. Na+ and K+).
The derivatization of the aqueous and acid extracts of the wound dressings with fluorescamine gave the MALDI mass spectra shown in Fig. 4 and 5. In both cases the peak corresponding to (M + K)+ in each family of peaks was not observed, neither was an increase corresponding to the addition of one or more fluorescamine molecules.
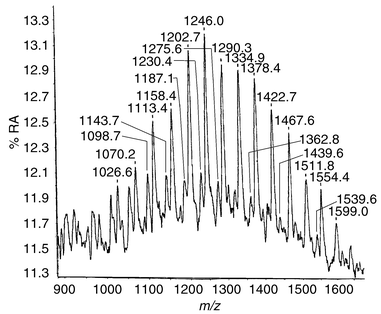 | ||
| Fig. 4 MALDI mass spectrum of aqueous extract derivatized with fluorescamine. | ||
 | ||
| Fig. 5 MALDI mass spectrum of acid extract derivatized with fluorescamine. | ||
The reason for the loss of the (M + K)+ peaks is unclear at this stage. However, the absence of peaks at M + 278, M + 556, etc., suggests that no amine functionality was present in the extracts within the detection limits of the experiment.
Conclusions
MALDI-MS can be used effectively to analyze extracts from polyurethane foam wound dressings, by providing a useful means of obtaining the molecular weight distribution of some polymeric materials.Furthermore, MALDI-MS in conjunction with fluorescamine labelling can successfully demonstrate the presence or otherwise of amine functionality in the extracts.
Similarly, reaction with phenyl isocyanate can give an indication of the functionality of polyols.
References
- D. Vitalini, P. Mineo and E. Scamporrino, Macromolecules, 1997, 30, 5285 CrossRef CAS.
- G. Montaudo, M. Montaudo, C. Puglisi and F. Samperi, J. Polym. Sci., Polym. Chem. Ed., 1996, 34, 439 CrossRef CAS.
- M. Karas and F. Hillenkamp, Anal. Chem., 1988, 60, 2299 CrossRef CAS.
- R. J. Cotter, Anal. Chem., 1992, 64, 1027A CAS.
- P. O. Danis and D. E. Karr, Org. Mass Spectrom., 1993, 28, 923 Search PubMed.
- M. Dey, J. A. Castoro and C. L. Wilkins, Anal. Chem., 1995, 67, 1575 CrossRef CAS.
- U. Bahr, A. Depp, M. Karas and F. Hillenkamp, Anal. Chem., 1994, 64, 2866.
- H. Koutali, J. Chalom, M. Tod, R. Farinotti and G. Mahuzier, Anal. Chim. Acta, 1992, 266, 243 CrossRef.
- K. Jackson, R. Jennings and J. H. Scrivens, J. Am. Soc. Mass Spectrom., 1997, 8, 76 CrossRef.
- R. P. Losada, J. S. Gandara and P. L. Mahia, J. Chromatogr. Sci., 1992, 30, 267.
Footnote |
| † Presented at SAC 99, Dublin, Ireland, July 25–30, 1999. |
| This journal is © The Royal Society of Chemistry 2000 |
