MALDI-MS and colorimetric analysis of diisocyanate and polyol migrants from model polyurethane adhesives used in food packaging†
Graham Lawson*, Sally Bartram, Sylvia Fitchner and Elaine D. Woodland
Department of Chemistry, De Montfort University, The Gateway, Leicester, UK LE1 9BH
First published on UnassignedUnassigned7th January 2000
Abstract
The identification of the migrants, into food simulants, from a series of polyurethane adhesives used in the manufacture of plastic film laminates for use in common food packaging is described. Commercial materials, based on four different model adhesive systems, were prepared by an industrial collaborator. The MALDI-MS fingerprint patterns of the three polyether and one polyester polyol components of these adhesives were obtained for reference purposes. The decrease in the level of diisocyanate as a migrant versus time after lamination was confirmed by colorimetric measurements. The migration of the standard polyol samples through polyethylene pouches into water at 70 °C has been demonstrated and also the attenuation effect for different polyols. Cured laminates in the form of pouches were used to carry out the migration experiments into distilled water, inside the pouch, at 70 °C over a period of 2 h. Comparison of the migration results from the food packaging laminates with those from the polyethylene film confirmed the migration of unreacted polyol components for the polyether-based systems. Cyclic oligomers from the polyol starting materials were identified as the migrants from the polyester-based adhesive.
Introduction
There is considerable current concern about the safety of food and its packaging. Plastic packaging is closely regulated for use in food contact applications,1–3 but there is currently no UK or EU legislation relating specifically to adhesives used in this area. The finished item would however be subject to the general provisions of the Materials and Articles in Contact with Food Regulations.1 There has been little work reported on the analysis of migrants from food packaging adhesives into food simulants. Gruner and Piringer4 have carried out quantitative studies on migrants from adhesives used in the paper and board packaging sector, but no chemical compounds were identified. The gravimetric determination of the overall migrants from polyurethane-based laminates into several food simulants has been reported by Lawson et al.5This investigation has concentrated on the analysis of migrants from a range of polyurethane (PU) adhesives used to bond two films together to form a laminate. Laminates are required when an individual film cannot meet the demands of the foodstuff or storage and preparation requirements. For example, polyethylene (PE) is often used as it can be heat-sealed, but it offers limited odour, flavour and gas barrier properties, whereas polyethylene terephthalate (PET) offers good gas barrier properties. Laminates of two such materials are used as bags for ground coffee, packs for smoked salmon, pouches for alcoholic cocktails and to package frozen boil-in-the-bag meals.
Matrix-assisted laser desorption/ionisation mass spectrometry (MALDI-MS) is a relatively new soft ionisation technique capable of looking at sample mixtures over a mass range of 150–500000 Da without prior separation. It has been applied over the past 5 years to the analysis of synthetic polymers because of its ability to measure oligomeric distributions. MALDI generally produces singly charged ions and facilitates the measurement of extremely high molecular weights with virtually no fragmentation and enables determination of repeat units and end group composition.6,7 This technique was used to identify the principal components of the species migrating from the PU adhesives.
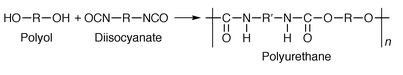
PU adhesives are reactive systems produced by the addition reaction of a polyol and a polyisocyanate:
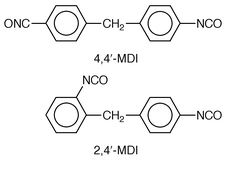
PU adhesives used in food packaging in the UK are usually derived from diphenylmethane diisocyanate (MDI)
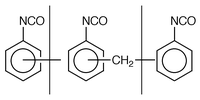
Common solvent-free adhesive formulations contain a mixture of the different monomers in solution in polymeric MDI (PMDI) which has a general structure:
Approximately 2400 tonnes of PU adhesive are used annually in Europe for solvent-free laminations, whilst another 2200 tonnes8 of the adhesive are used for similar purposes, but are prepared in solution in either a ketone or ethyl acetate.
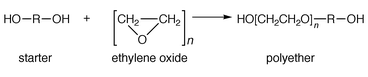
The polyol component of the PU adhesive can be either a polyfunctional polyether or polyester, a mixture of both or castor oil. In all cases, for use in flexible adhesives the molecular mass of the polyol component is in the range 400–2000. Polyether polyols are produced by the addition of either ethylene oxide or propylene oxide to a polyhydroxy ‘starter’ molecule in the presence of a catalyst:
Typical starter molecules include glycerol, ethylene glycol, propylene glycol and trimethylolpropane.
Polyester polyols are produced by the condensation reaction of polyfunctional carboxylic acids or anhydrides with polyfunctional alcohols
| HOOC–R–COOH + HO–R′–OH ⇌ carboxylic acid alcoholHOOC–R–COO–R′ –OH + H2Oester | (1) |
In commercial applications it is common to find polyesters prepared from a mixture of two or more acids reacted with two or more glycols, which gives scope for a range of very complex products. Typical materials used in a single ‘simple’ commercial polyol formulation include diethylene glycol, neopentyl glycol, adipic acid and isophthalic acid. Both the polyether and polyester polyol components will therefore contain a range of oligomeric material and also any stabilisers, pH adjusters and other additives, any of which may be potential migrants once the laminating process has been completed.
Clearly, a PU adhesive is a complex material which starts as a mixture of the diisocyanate and free polyol as the bonding layer between two plastic films. This mixture cures with time and hence the species available for migration will change from the original starting materials to final reaction products and residues. The Flexible Packaging Association (FPA), the trade association representing the manufacturers of this type of packaging, have a code of practice which recommends that all such PU bonded laminates are stored for 7 d prior to dispatch. This delay after lamination ensures that the residual diisocyanate level has decayed to less than 1 ppm in order to meet the current plastics regulations.2
Experimental
Migration experiments were carried out using commercially prepared PET–PU–PE films heat-sealed into pouches. The food simulants were placed in the pouch and migration into the pouch contents was investigated. In the course of this study using model commercial PU adhesives, the level of migratable diisocyanate as a function of time after lamination was studied to determine the time after which the material would be considered cured (<1 ppm diisocyanate). A MALDI-TOF mass spectrometer and colorimetric methods were used to study the species migrating into different food simulants from the cured laminate. Experiments were also carried out to confirm the poor barrier properties of the PE film to liquid polyols.Packaging samples
All the laminates used in this investigation were prepared commercially using 12 μm PET bonded to 45 μm low-density PE with a range of different PU adhesives. The commercial preparation of the samples ensured the quality of the laminate, particularly the correct adhesive coat weight within the range 1–2.5 g m−2 as appropriate to each adhesive formulation. The adhesives were simplified versions of commercial products based on monomeric and polymeric MDI with known polyol formulations. The samples were supplied by industrial collaborators.Migration experiments
Pouch samples (20 × 20 cm) were prepared by heat-sealing the laminate with the PE as the inner layer next to the different food simulants. Similar pouches, made from the PE film used as the inner layer of the laminate, were prepared for the liquid polyol migration studies. The food simulant used was Millipore (18 MΩ) water. The pouch laminates containing 100 cm3 of food simulants were placed in the oven for 2 h at 70 °C. After this time the simulant was removed and concentrated to 10 cm3 using a rotary evaporator at 60 °C. For the polyol migration studies the simulant was the water surrounding the pouch.Reagents
The following reagents were used: Millipore water (resistivity 18 MΩ cm, Milli-RO15 water system); analytical-reagent grade acetic acid and hydrochloric acid, N-(1-naphthyl) ethylenediamine dihydrochloride (NEDD), 4,4′-methylenedianiline (MDA) and aniline hydrochloride (Fisher Scientific, Loughborough, Leicestershire, UK); sodium nitrite (Sigma-Aldrich, Gillingham, Dorset, UK); ammonium sulfamate (Merck/BDH, Lutterworth, Leicestershire, UK); and polypropylene oxide (ICI Surface Coatings, Birmingham, UK).Colorimetric determination of primary aromatic amines
A Unicam UV-2-100 UV/VIS spectrometer, equipped with a 40 mm pathlength (silica) cell and operated at a wavelength of 550 nm, was used.The following solutions were used:
Under the experimental conditions any migrating diisocyanate would be hydrolysed to the corresponding diamine, which can then be detected colorimetrically using a diazotisation reaction based on the work of Marcali.9,10 Freshly prepared laminate, less than 4 h after lamination, was collected from the converters, returned to the laboratory and subjected to the migration experiments detailed above on a twice daily basis for 14 d. After extraction, the aqueous food simulant was removed from the pouch and rotary evaporated under vacuum at 60 °C to 10 cm3; the derivatisation step was then carried out as follows: 5 cm3 of acid solution and 2 cm3 of acetone were added to the concentrated simulant; 1 cm3 of sodium nitrite solution was added and left for 10 min. After this, 2 cm3 of ammonium sulfamate were introduced and left to react for a further 10 min; then, 2 cm3 of coupling reagent were added and the colour was allowed to develop for 2 h before measurement at 550 nm. These measurements were replicated and the concentration of MDA was determined from a calibration graph prepared from authentic MDA. A simple calculation allowed the mass of the migrating MDI to be calculated from the measured mass of MDA.
MALDI-MS analysis
A Finnigan Lasermat-2000 linear time-of-flight mass spectrometer, equipped with a N2 laser pulsed at 337 nm, was used.MALDI-MS only requires 1 μl of sample (concentration 10−5–10−7 M) to be dried with a selected matrix and placed in the ion source. A UV laser then volatilises/ionises the sample and matrix; ionisation occurs during volatilisation by energy transfer between matrix and analyte. Gentisic acid (2,5-dihydroxybenzoic acid) was employed as the matrix in this investigation. A 1 μl aliquot of sample was mixed with 1 μl of matrix (concentration 1 g l−1 in water) and dried for analysis.
Results
Residual isocyanate moiety expressed as the amine
The results obtained (Fig. 1) show that the migratable isocyanate level reaches 1 ppm approximately 50 h after lamination. The FPA recommended time delay is shown on Fig. 1 to indicate the anticipated migratable NCO level from a typical commercial laminate. These results show that the level of NCO moiety migrating from the laminate is well below that expected from the EU specified residual level of 1 ppm free NCO moiety.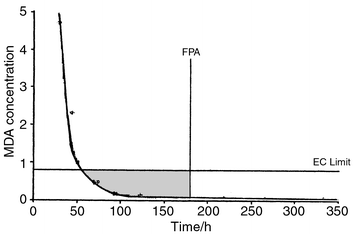 | ||
| Fig. 1 MDA concentration in a food simulant versus time after lamination for a commercial laminated pouch. | ||
MALDI-MS analysis of polyol standards
The mass spectra of the polyether samples (mean molecular weights 500, 1000 and 2000) showed a clear distribution of oligomers centred on the expected molecular weight; see for example the spectrum from polyol 2000 (Fig. 2).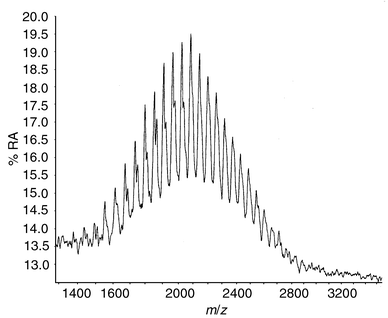 | ||
| Fig. 2 MALDI mass spectrum of a liquid polypropylene oxide polyether polyol (Mw 2000). (%RA = per cent relative abundance) | ||
The spacing between peaks of 58 units corresponds to a propylene oxide [CH2CH2(CH3)O] unit and the shoulders on the low mass peaks result from the sodium adduct ions (M + 23)+ of the corresponding oligomer. The spectrum of a simple polyester polyol based on adipic acid (AA) and diethylene glycol (DEG) showed a much wider mass range, 500–5000, due to the (AA–DEG) repeat unit with a mass of 216. Furthermore, the molecular weight distribution was asymmetric with a long tail at high mass. These traces were used as reference data for the subsequent migration experiments.
Migration through PE films
MALDI analysis (Fig. 3) of the components of polyols which can migrate through a 45 μm PE film demonstrates that bifunctional polypropylene oxide systems with molecular weights up to 1600 can readily migrate with little change in the relative abundance of the oligomers. Trifunctional polyether systems (Fig. 4) show a much lower potential for migration (limit about 600 mass units) and there is a clear change in the relative abundance of the oligomers after passage through the film. There is a similar attenuation of the migrating oligomers from the polyester polyol. Fig. 5 compares the full range of oligomers (upper trace) with those capable of passage through the PE film (lower trace). These results clearly demonstrate that the PE film used in commercial laminates presents little barrier to polyol migration where the liquid material is present.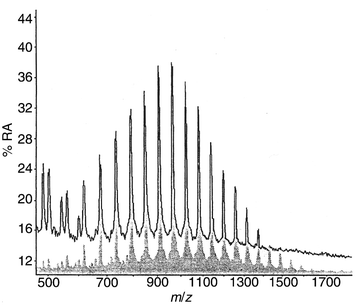 | ||
| Fig. 3 MALDI mass spectrum of liquid bifunctional polypropylene oxide (Mw 1025) before (top trace) and after (bottom trace) migration through 45 μm low-density PE film into water. | ||
 | ||
| Fig. 4 MALDI mass spectrum of liquid trifunctional polypropylene oxide (Mw 440) before (top trace) and after (bottom trace) migration through 45 μm low-density PE film into water. | ||
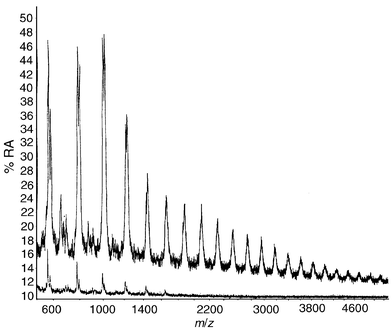 | ||
| Fig. 5 MALDI mass spectrum of a simple liquid polyester polyol, before (top trace) and after (bottom trace) migration through 45 μm low-density PE film into water. | ||
Migrants from commercial pouch samples
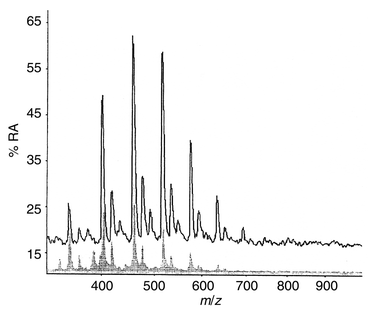
For commercial samples composed of polyether polyols, the MALDI mass spectra are similar to, or identical with, those shown in Fig. 2, whereas for the polyester polyols the MALDI data did not correspond to those shown for the liquid polyol. The migrants from the polyester-based laminate showed no detectable evidence of the anticipated oligomers. The only migrants observed (Fig. 6) have been identified, by synthesis and LC-MS analysis, as cyclic oligomers which have no hydroxyl functionality and therefore cannot be ‘locked into’ the polymer backbone by reaction.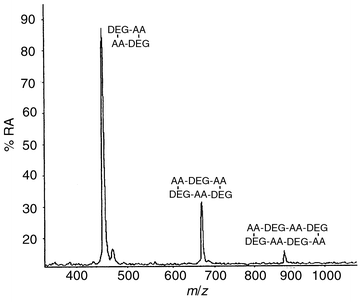 | ||
| Fig. 6 MALDI mass spectrum of the migrants from a laminate bonded with a PU containing a simple polyester-based polyol. | ||
Conclusions
MALDI-MS provided rapid meaningful analyses of both the reference polyol sample and the products of migration.The only MALDI-MS detectable species present in the migrants from PU-based adhesives are oligomers from the polyol component of the adhesive. This is a surprising result since the PU formulations are used with an excess of diisocyanate which is consumed by atmospheric moisture during the curing process. Hence, some polyol must dissolve in the PE film prior to reaction and this is isolated from the diisocyanate and is therefore free to migrate. This argument does not however explain why no linear polyester oligomers are observed.
MALDI-MS measurements cannot readily be quantified and therefore a derivatisation methodology must be developed to facilitate the HPLC quantification of the levels of polyol migration.
Acknowledgements
S.F. was financed under the Socrates scheme. Financial support for S.B. from the Ministry of Agriculture, Fisheries and Food is gratefully acknowledged.References
- EEC Directive 89/109, Off. J. Eur. Comm., 1989, L40, 38..
- EEC Directive 90/128, Off. J. Eur. Comm., 1990, L349, 26..
- EEC Directive 92/39, Off. J. Eur. Comm., 1992, L168, 21..
- A. Gruner and O. Piringer, Packag. Technol. Sci., 1999, 12, 19 CrossRef CAS.
- G. Lawson, C. T. Barkby and C. Lawson, Fresenius’ J. Anal. Chem., 1996, 354, 483 CAS.
- H. J. Rader and W. Schrepp, Acta. Polym., 1998, 49, 272 CrossRef.
- P. Zollner, G. Stubinger, F. Schmid, E. Pittenauer and G. Allmaier, Int. J. Mass Spectrom. Ion Processes, 1997, 169, 99 CrossRef.
- T. Cadby, ICI Surface Coatings, personal communication..
- K. Marcali, Anal. Chem., 1957, 29, 552 CrossRef CAS.
- C. T. Barkby, PhD Thesis, De Montfort University, 1995..
Footnote |
| † Presented at SAC 99, Dublin, Ireland, July 25–30, 1999. |
| This journal is © The Royal Society of Chemistry 2000 |