Rapid analysis of illicit drugs by mass spectrometry: Results from seizures in Ireland†
Donnacha O’Connell and James J. A. Heffron*
Analytical Biochemistry and Toxicology Laboratory, National University of Ireland, Lee Maltings, Prospect Row, Cork, Ireland.. E-mail: j.heffron@ucc.ie
First published on UnassignedUnassigned24th December 1999
Abstract
A gas chromatographic procedure with mass spectrometric detection (GC-MS) was established to determine the principal amphetamines, methylenedioxymethylamphetamine (MDMA), methylenedioxyethylamphetamine (MDEA) and methylenedioxyamphetamine (MDA), cocaine and pharmacologically active impurities in ‘ecstasy’ tablets. The procedure was developed and optimised by combining the individual methods for the various drugs of abuse available in the literature into a single GC-MS run. New variants of the main drugs which may appear on the drug scene were also detected, including N-methyl-1-phenylethylamine, a member of a new series of illicit drugs. The method is rapid and has good sensitivity and reproducibility.
Introduction
Amphetamine and its derivatives including ecstasy and related compounds are major drugs of abuse. Although patented in 1914 as an appetite suppressant,1 methylenedioxymethylamphetamine (MDMA), commonly referred to as ‘ecstasy’, is a relatively new drug of abuse. It is widely known as ecstasy because of the euphoria it produces in its users. MDMA is a hallucinogen as well as a stimulant and appetite suppressant. MDMA in illicit drug preparations was first observed in 1972 and various aspects of its pharmacology have been reviewed.2,3 Ecstasy is reported to have the unique ability to facilitate interpersonal communication by reducing the anxiety and fear that normally accompanies the discussion of painful events.4Illicit drugs are rarely 100% pure. Until recently the ecstasy group consisted of three substances: MDMA, methylenedioxyethylamphetamine (MDEA) and methylenedioxyamphetamine (MDA), with the typical drug content being close to 100 mg per tablet.5 Ecstasy is usually found in tablet form with a distinctive ‘logo’ such as a dove, a shamrock, a cartoon character or some other emblem. The term ecstasy is now used so widely that it may be considered to be virtually generic for any member of the ring-substituted amphetamine group which includes 178 compounds published in PiKHAL6 including 2,5-dimethoxy-4-bromoamphetamine (DOB) and 4-methyl-2,5-beta-trimethoxyamphetamine (BOD). Other related compounds such as 4-methylthioamphetamine (4-MTA) are also categorised in the media and on the street in the ‘ecstasy’ group of compounds. DOB and 4-MTA or ‘flatliners’ as they are called have been discussed in the popular media recently. 4-MTA is believed to have been responsible for at least four deaths in the United Kingdom in the last year alone. While DOB is considered 33 times more potent than MDMA, with an active dose of only 2–4 mg, it should not be confused with BOD which has very little effect up to 75 mg.
Chromatography has played a dominant role in forensic drug chemistry over the past 30 years. When a mass spectrometer is used as a GC detector the main advantage is the potential to characterise structurally the impurities and by-products. A detailed review of chromatographic methodology for illicit refined cocaine including the development of cocaine impurity signature profiles has been published.7 Many papers have also been published on amphetamine analysis. 8,9
While there are several GC-MS methods available in the literature for separating individual groups of drugs, there did not seem to be any multi-drug and impurities procedure. Our procedure was optimised by combining the individual methods available in the literature into a single analytical run that could detect amphetamines, cocaine, active impurities and new illicit drugs such as N-methyl-1-phenylethylamine.
Experimental
Chemicals
Standard preparations of MDMA, MDEA and MDA were synthesised by Dr. M. O’Sullivan, Chemistry Department, National University of Ireland, Cork, under licence from the Irish Department of Health. No impurities were detected in these standards by GC-MS or by NMR. DL-Amphetamine and cocaine hydrochloride were obtained from Sigma-Aldrich (Poole, Dorset, UK). R-(+)- and S(−)-N-methyl-1-phenylethylamine were obtained from Fluka Laboratory Chemicals, Sigma-Aldrich Ireland (Tallaght, Dublin, Ireland). Absolute ethanol was supplied by Carbery Distillers. (Co. Cork, Ireland).Four randomly chosen amphetamine powders and 61 tablets confiscated as ‘ecstasy’ from 13 seizures by the Garda Siochána (Irish police force) in the period 1995–98 were analysed.
Sample preparation
All drug samples and standards were prepared in absolute ethanol. Tablets, ranging in mass from 248 to 322 mg, were initially ground into powders. A 20 ml volume of absolute ethanol was then added to each powder. The solution was filtered to remove insoluble compounds and 0.2 ml of the filtrate was made up to 1.0 ml with absolute ethanol. This procedure gave an approximate concentration of 1 mg ml−1 MDMA based on the average MDMA content of ecstasy tablets.Gas chromatography-mass spectrometry
The GC-MS system (Hewlett-Packard, Rockville, MD, USA) consisted of a Model 5890 Series II gas chromatograph, an ENGINE Model 5989A mass spectrometer (electron ionisation/chemical ionisation model), a Model 7673 auto-injector and a Chemstation computer system. The column used was an HP1 fused-silica capillary column (60 m × 0.25 mm id) with a 1 μm film thickness of methylsilicone. Injections were carried out in the splitless mode (injection volume 1 μl ). The carrier gas was high-purity helium (BOC Gases, Cork, Ireland) at a flow rate of 1 ml min−1 . The mass spectrometer was used in the EI (electron ionisation) mode, the source temperature was 200 °C, the quadrupole temperature was 100 °C and the electron energy was 70 eV. Mass spectra were obtained by scanning from 35 to 400 m/z at 1.42 s per scan. The injector and interface temperatures were maintained at 250 and 290 °C, respectively.The initial oven temperature was 190 °C; this was held for 4 min followed by a linear ramp to 250 °C at 20 °C min−1; this was held for 5 min and followed by a linear ramp to 290 °C at 30 °C min−1; the final temperature was held for 20 min. The total analysis time was 33 min. The peaks on the chromatograms were quantified by area.
Precision
The precision of the procedure was tested by analysing a standard of MDMA ten times, which gave a relative standard deviation of 5.5%.Sensitivity
The method gave a limit of detection of 32 ng per injection for MDMAResults
The results of the GC-MS procedure for separating amphetamine, cocaine and active impurities found in ecstasy tablets are illustrated in a chromatogram (Fig. 1). It is evident that separation satisfactory for quantification was achieved. Typical amphetamine samples were found to contain butanoic acid, caffeine, dimethylformamide and pseudoephedrine as well as amphetamines. The mere presence of impurities in an amphetamine profile shows that the material is illicit as pharmaceutical-grade amphetamine has few, if any, detectable contaminants. The purity of the amphetamine was found to be 12%, which is purer than amphetamine found in a recent study in the UK.5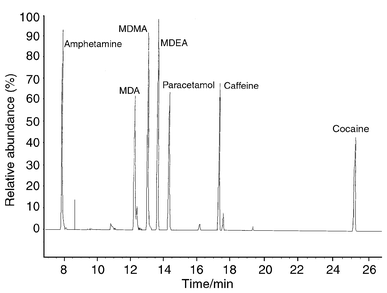 | ||
| Fig. 1 Example of the separation of a range of drugs of abuse. | ||
Fig. 2 shows a chromatogram of a ‘Dove’ confiscated in 1995 which was found to contain 130 mg of MDMA and a trace amount of MDEA at 13.6 min. The mass spectrum of the MDMA is also shown in Fig. 2 with the molecular ion peak at m/z 193 and the characteristic peak at m/z 58. A ‘Dove’ tablet confiscated in 1996 contained 65 mg of MDEA and 20 mg of MDMA whilst a ‘Smiling Sun’ logo tablet confiscated in 1998 contained 4 mg of MDEA and 100 mg of an unknown component subsequently identified as N-methyl-1-phenylethylamine (N-MePEA). The unknown component had a retention time of 7.2 min and was present in many of the ecstasy tablets which were analysed. This compound was not in the Wiley or NIST libraries. The unknown compound was identified by reference to a published spectrum10 and by co-injection as N-methyl-1-phenylethylamine. N-MePEA was found in many of the samples described as ‘ecstasy’, an example of which is given in Fig. 3. This tablet contained no MDMA; it was mainly caffeine with 11.5 mg of amphetamine and also N-MePEA. These combinations have also been found in UK seizures.10
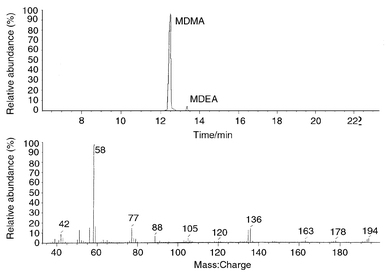 | ||
| Fig. 2 A chromatogram of a ‘Dove’ confiscated in 1995. | ||
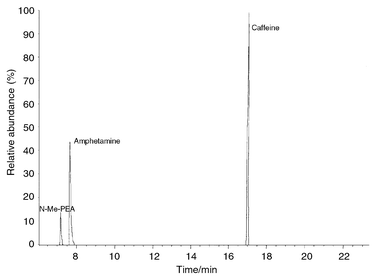 | ||
| Fig. 3 A chromatogram of a ‘Dove’ confiscated in 1997. | ||
The results of the analyses of recent ‘ecstasy’ seizures are summarised in Table 1.
| Compound | Major occurrence, ![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) 30 mg per
tablet 30 mg per
tablet | Minor occurrence, <30 mg per tablet |
|---|---|---|
| MDMA | 9 | 2 |
| N-MePEA | 5 | 2 |
| MDEA | 1 | 3 |
| Amphetamine | 1 | |
| MDA | 1 | 1 |
| Aspirin | 1 | |
| Caffeine | 1 | |
| Paracetamol | 1 | |
| Pseudoephedrine | 1 |
Discussion
The GC-MS procedure described here enables an easy, straightforward and rapid screening of an unknown illicit drug sample to be performed in less than 1 h. The sensitivity of the procedure permits detection of MDMA in tablets with as little as 1% amphetamine.Impurities are often observed in illicitly prepared drug samples and the identification of the reaction intermediates in the illicit sample can be used to establish the synthetic route used. Some impurities may be biochemically active compounds. Knowledge of these impurities is important for several reasons. It may link samples to a common source, dealer or illicit laboratory, and the toxicity of these impurities may have potential harmful effects on the drug users. Drug purities may also give useful information on the economic behaviour of the illicit market.
From our studies of recent ‘ecstasy’ seizures in Ireland the amount of MDMA in tablets classified as ecstasy can vary from not detectable to 180 mg per tablet. Many active compounds were also present in the ecstasy tablets, including caffeine, amphetamine, MDEA, ephedrine/pseudoephedrine and N-MePEA (Table 1). The composition of ecstasy tablets may vary dramatically as illicit drug manufacturers do not have a quality control policy. The “logos” on the tablets are not very reliable indicators of composition as tablets with the same logo have very variable amounts of the same active ingredients, as can be seen from the examples above.
N-Methyl-l-phenylethylamine, a relatively new designer drug, was found in about one-third of Irish seizures referred to us. The pharmacological effects of these phenylethylamines appear to be unknown as there are no published studies on the biochemistry, pharmacology or toxicology of N-MePEA in humans.10
Acknowledgements
The authors acknowledge Enterprise Ireland for their financial support as part of the Irish Government’s Science and Technology against Drugs Initiative, Dr. Mary O’Connor of the Forensic Science Laboratory, Dublin, Superintendent Eddie Rock of the National Drugs Unit, Dt. Ins. Tony Quilter and Dt. Garda Mary King of the Garda Siochána Drug Squad for supplying the drug samples under licence.References
- E. Merck, Ger. Pat., 274 350, 1914..
- A. T. Shulgin, J. Psychoactive Drugs, 1986, 18, 291 Search PubMed.
- G. N. Hayner and H. McKinnonf, J. Psychoactive Drugs, 1986, 18, 341 Search PubMed.
- A. T. Shulgin and D. E. Nicholas, in The Psychopharmacology of Hallucinogens, ed. R. C. Stillman and R. E. Willett, Pergamon Press, New York, 1987, p. 74. Search PubMed.
- L. A. King, Forensic Sci. Int., 1997, 85, 135 CrossRef CAS.
- A. Shulgin and A. Shulgin, PiHKAL: The Chemical Story, Transform Press, Berkeley, CA, 1991, Search PubMed.
- J. M. Moore and J. F. Casalef, J. Chromatogr. A, 1994, 674, 165 CrossRef CAS.
- Special Issue, Forensic Sci. Int., 1994, 69, No. 1. Search PubMed.
- S. Alm, I. Granstam, S. Jonson and L. Strömberg, Classification of Illegal Leuckart Amphetamine by Gas Chromatographic Profiling, Report 25, Statens Kriminaltekniska Laboratorium, SKL,S-581 01, Linköping, Sweden, 1992. Search PubMed.
- L. A. King, A. J. Poortman-Van Der Meer and H. Uizerf, Forensic Sci. Int., 1996, 77, 141 CrossRef CAS.
Footnote |
| † Presented at the SAC 99 Meeting Dublin, Ireland, July 25–30, 1999. |
| This journal is © The Royal Society of Chemistry 2000 |
