Design of conjugated molecular materials for optoelectronics†
Takeshi Sano‡*, Yoshitaka Nishio, Yuji Hamada, Hisakazu Takahashi, Tatsuro Usuki and Kenichi Shibata
New Materials Research Center, Sanyo Electric Co., Ltd., 1-18-13 Hashiridani, Hirakata-City, Osaka 573-8534, Japan, ts233@cus.cam.ac.uk
First published on UnassignedUnassigned22nd December 1999
Abstract
Fluorescent metal complexes and dimers have been synthesized and used for organic light-emitting diodes. The metal complexes with conjugated molecular ligands have strong photoluminescence, and showed bright emission of over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 in bilayer electroluminescent (EL) cells. The EL cell structure was [transparent anode/hole-transport layer/emitting layer/cathode]. The newly synthesized pyrazoline dimers are also highly fluorescent, and they have hole-transport tendency. Therefore, another type of structure [transparent anode/emitting layer/electron-transport layer/cathode] was applied to get emission from the pyrazoline derivatives. As a result, one pyrazoline derivative showed bright blue EL of 1700 cd m−2 at the maximum when it was combined with an oxadiazole electron-transport material.
000 cd m−2 in bilayer electroluminescent (EL) cells. The EL cell structure was [transparent anode/hole-transport layer/emitting layer/cathode]. The newly synthesized pyrazoline dimers are also highly fluorescent, and they have hole-transport tendency. Therefore, another type of structure [transparent anode/emitting layer/electron-transport layer/cathode] was applied to get emission from the pyrazoline derivatives. As a result, one pyrazoline derivative showed bright blue EL of 1700 cd m−2 at the maximum when it was combined with an oxadiazole electron-transport material.
1 Introduction
Electroluminescence (EL) and charge transport in organic thin films have attracted strong interest because of their potential use for organic light-emitting diodes (OLEDs) and thin film transistors (TFTs). The materials and the fabrication technique for OLED displays have progressed remarkably in the past ten years, and it has been announced that new flat panel displays with this technology will be on the market soon.1,2Electroluminescence of an organic material was first reported in the 1960s with anthracene crystals,3 but the quantum efficiency was not good enough for practical use. A breakthrough was made in 1987 by Tang and VanSlyke4 at the Eastman Kodak Co. They made a thin-film device with two sublimed molecular layers sandwiched between the electrodes, and obtained bright green emission through the transparent anode. It was a innovation to make a very thin bilayer structure with two different organic thin films, and to use two electrodes of different work functions which are capable of injecting carriers into the organic layers.
After this report, organic electroluminescent materials and devices were studied extensively.5–10 A large variety of fluorescent molecules available in vacuum vapor deposition have been explored. Small molecules have the advantages of ease of purification, processability and variety, but disadvantages of low efficiency and stability. However, the efficiency and stability of OLEDs have been improved by incorporation of highly fluorescent molecules.11–15 In addition, energy band studies of EL devices have helped to optimize their performance.16–18 As a result, the first OLED display was commercialized1 in 1997.
Another active area of organic-based EL research is the ‘Light Emitting Polymer (LEP)', which was first reported by Burroughes et al.19 in 1990. Polymers are thought to be more stable than small molecules. Processability and efficiency were problems in the early studies. However, they have been improved remarkably by optimization of both polymer design and devices.2,20
We have concentrated on the studies of small molecules, because they are easy to handle in conventional vacuum deposition equipment. There is a large variety of small fluorescent molecules but not so many molecules that can make uniform thin films in vacuum vapor deposition. If a small molecule can be sublimed in vacuum successfully, it often forms a polycrystalline film with many pinholes, which leads to device failure. Even when a uniform thin film is obtained, carrier transport ability and fluorescent yield of the film are the next problems. Knowing the energy bands of the HOMO and LUMO levels for a compound is also important in fabricating an organic EL cell. To summarize, the following properties are strongly required or indispensable for the EL materials: (1) form a uniform thin film; (2) carrier (hole/electron) transport ability; (3) high fluorescent yield; (4) stable to heat (have a high glass-transition temperature); (5) suitable HOMO/LUMO levels for the carrier injections.
A certain direction of molecular design is required to obtain uniform and stable organic thin films. In the following section several kinds of EL materials available in vacuum vapor deposition are discussed.
1.1 Quinolinolato and benzoquinolinolato complexes
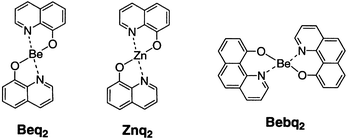
The most popular molecule for OLED is the 3:1 complex of 8-hydroxyquinoline aluminum salt (Alq3) which was reported by Tang and VanSlyke.4We have found several kinds of 2:1 complexes which also have strong fluorescence and are sublimable in vacuum.8 We made 2:1 complexes with 8-hydroxyquinoline and a variety of metal ions, such as Be, Mg, Ca, Sr, Sc, Y, Cu, or Zn. Amongst these, the beryllium complex (Beq2) was found to be the most fluorescent. Other complexes with heavy metals, for example, the copper complex, are less fluorescent. However, the zinc complex (Znq2) was found to have a strong yellow fluorescence, although zinc is a relatively heavier metal than beryllium.
A 2:1 complex with 10-hydroxybenzo[h]quinoline and beryllium (Bebq2) was also synthesized.9 This complex was found to have a very strong green fluorescence and a high melting point (368![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C) which are needed in EL materials.
°C) which are needed in EL materials.
1.2 Azomethine–zinc complexes
As the quinolinol complexes have fluorescence in the green to yellow region, another type of emitting material is needed for blue emission. Newly synthesized materials for blue electroluminescence are the 2:1 and 1:1 azomethine–zinc complexes bis(N-methylsalicylideneamino)zinc(II) (2AZM-Me) and (N, N′-disalicylidenehexane-1,6-diaminato)zinc(II) (1AZM-Hex), respectively.7 These compounds were also sublimable. A few other asymmetric complexes were synthesized as reported previously, but they decomposed before subliming. These azomethine–zinc complexes have strong blue fluorescence and high melting points.1.3 Thenoyltrifluoroacetonato (TTA) europium complex
In the search for suitable red electroluminescent materials, europium complexes, which in general display fluorescence in the red region, have been investigated. We have found that a europium complex with four chelating ligands is sublimable where the fourth ligand is a neutral molecule such as phenanthroline (phen):21 (1,10-phenanthroline)tris(thenoyltrifluoroacetonato)europium(III) (Eu(TTA)3(phen)). This complex has an intense red fluorescence with a sharp spectral bandwidth even in the solid state.1.4 Benzothiazolate zinc complex
A greenish white fluorescent molecule has been also synthesized:22 bis(2-(2-hydroxyphenyl)benzothiazolato)zinc (Zn(BTZ)2). This complex has a wide fluorescent bandwidth from 430 to 700 nm in the photoluminescence (PL) spectrum.2 Experimental
The chemicals were purchased from Wako Chemicals Co. The complexes were synthesized in ethanol–aqueous solutions and precipitated. They were filtered off, dried and purified in train sublimation. The details were given in previous reports.7–9,21,22The EL devices were fabricated as follows. The indium–tin oxide (ITO) coated glass substrates were patterned and washed. On the substrate, the hole-transport layer and the emitting layer were deposited successively under a high vacuum (10−3−10−4 Pa). The cathode was deposited on the top through a shadow mask. The cross-section view of the EL cell is shown in Fig. 1. For the hole-transport layer, N,N′-bis(3-methylphenyl)-N,N′-diphenyl-1,1′-biphenyl-4,4′-diamine (TPD) was used. The newly synthesized metal complexes were used for the emitting layer and magnesium and indium alloy for the cathode metal. The typical thicknesses of the layers were: hole-transport layer, 500; emitting layer, 500; and cathode, 2000 Å. The size of the each cell was 2 × 2 mm.
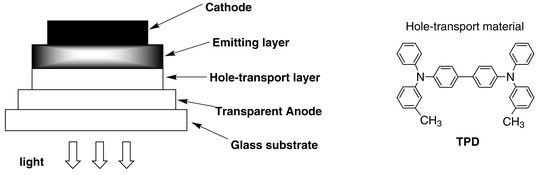 | ||
| Fig. 1 The EL cell structure and the molecular structure of TPD. | ||
The PL and EL spectra were measured with a multichannel spectrometer of Otuka Electronics. The luminance–current–voltage (L–I–V) characteristics were measured with a Topcon luminance meter and a Keithley digital multimeter.
3 Results and discussion
3.1 Metal complexes
8-Hydroxyquinolinatometal complexes showed very high luminance in the EL cells as shown in Table 1. Beq2 showed green emission of 8700 cd m−2 at 20 V, and Znq2 showed yellow emission of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 cd m−2 at the same voltage. Bis(10-hydroxybenzo[h]quinolinato)beryllium (Bebq2) was the most brilliant, showing intense green emission of 19
200 cd m−2 at the same voltage. Bis(10-hydroxybenzo[h]quinolinato)beryllium (Bebq2) was the most brilliant, showing intense green emission of 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 at 16 V. The EL spectra of these complexes were almost the same as their PL spectra, which indicates the EL originated from the complexes.
000 cd m−2 at 16 V. The EL spectra of these complexes were almost the same as their PL spectra, which indicates the EL originated from the complexes.
| Complex | PL peak wavelength/nm | Emission color | Maximum luminance/cd m−2 | Efficiency/cd A−1 |
|---|---|---|---|---|
| Beq2 | 520 | Green | 8700@20 V | 3.10 |
| Znq2 | 556 | Yellow | 16 200@20 V | 3.23 |
| Bebq2 | 515 | Green | 19 000@19 V | 6.13 |
| 2AZM-Me | 439 | Blue | 420@22 V | 0.17 |
| 1AZM-Hex | 450 | Blue | 1460@16 V | 1.58 |
| Eu(TTA)3(phen) | 614 | Red | — | — |
| Zn(BTZ)2 | 524 | Greenish white | 10 190@8 V | 1.39 |
Azomethine–zinc complexes showed pure blue emission, which is precious in fabricating multi-color OLED displays. The EL peak wavelength of 2AZM-Me was shifted to 461 nm, which indicates an exciplex was made at the TPD/2AZM-Me interface. The maximum luminance of 2AZM-Me was less than expected because of the exciplex. On the other hand, the EL peak wavelength of 1AZM-Hex was 460 nm, relatively close to the PL one, and the EL cell showed bright blue emission of 1460 cd m−2 at the maximum.
The hydroxyphenylbenzothiazolate zinc complex also showed bright emission, a unique greenish white, which is thought to be useful in several practical applications. This white color was not from an exciplex but Zn(BTZ)2 itself. Fig. 2 shows the PL and EL spectra which were almost identical.
 | ||
| Fig. 2 Photoluminescence (broken line) and electroluminescence (solid line) spectra of Zn(BTZ)2. | ||
Eu(TTA)3(phen) was the only complex which did not show a visible emission among these metal complexes. Its PL mechanism is totally different from that of the other complexes, and originates in the transition between the energy levels of a europium(III) ion, as in the PL spectrum shown in Fig. 3. Therefore, it did not show emission in the same EL system. Other kinds of EL system should be applied for this europium complex.
 | ||
| Fig. 3 Photoluminescence spectrum of Eu(TTA)3(phen). | ||
In general, metal complexes which have conjugated molecular ligands could form uniform thin films in vacuum vapor deposition and are reasonably stable to heat which are needed for OLED displays. The present metal complexes showed bright emission when they were used for the emitting layer in the conventional bilayer structure, [transparent anode/hole-transport layer/emitting layer/cathode]. Some were found to be practically useful, for example, Znq2 for yellow, Bebq2 for green, 1AZM-Hex for blue and Zn(BTZ)2 for white emission.
3.2 Dimers
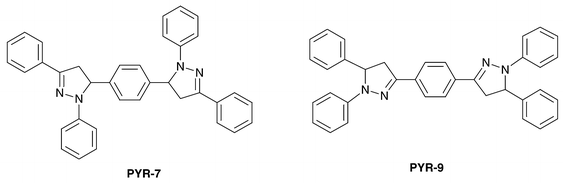
The most popular hole-transport molecule is TPD, which is a triphenylamine dimer. This compound is known as a carrier transport material in combination with an organic photoconductor (OPC), which can be used in a copying machine. TPD is also useful in OLEDs, so that we can learn from the molecular design of TPD. The dimer structure has stabilized this molecule. Few materials which have a better hole-transport ability than TPD are known.PYR-7 and PYR-9 are newly synthesized pyrazoline derivatives. Both compounds showed reasonably high melting points of between 250 and 260![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, and uniform amorphous films were obtained in vacuum vapor deposition. Strong PL was seen in both solids and films, which originated in the conjugated system including pyrazoline rings. The PL peak wavelength of PYR-7 was shorter than that of PYR-9, which means its conjugated system is smaller than that of PYR-9.
°C, and uniform amorphous films were obtained in vacuum vapor deposition. Strong PL was seen in both solids and films, which originated in the conjugated system including pyrazoline rings. The PL peak wavelength of PYR-7 was shorter than that of PYR-9, which means its conjugated system is smaller than that of PYR-9.
These two pyrazoline compounds were examined in the conventional EL cells. The EL cells of the same structure [transparent anode/hole-transport layer/PYR derivative/cathode] did not show visible emission. The reason why is that the pyrazoline compounds have hole-transport tendency basically in contrast with the metal complexes having electron-transport tendency.
In order to get better efficiency, an opposite type of EL cell structure was applied for the pyrazoline compounds. The new structure was [transparent anode/emitting layer/electron-transport layer/cathode], first introduced by Adachi et al.24 at Kyushu University. An oxadiazole derivative,6 OXD-7, was used for the electron-transport layer (Fig. 4). By using this structure, bright emission from pyrazoline compounds was obtained successfully. Table 2 shows the characteristics of the pyrazoline derivatives and the EL cells. The EL peak wavelengths were identical to the PL ones. Blue emission of 1700 cd m−2 was obtained from the PYR-7 EL cell, and blue-green emission of 5900 cd m−2 from the PYR-9 cell, respectively. In spite of the unusual EL cell structures, they showed very good efficiency. Fig. 5 shows the emission spectra of the EL cells in which PYR-7 and PYR-9 were used as emitting hole-transport layers respectively.
 | ||
| Fig. 4 The new type EL cell structure and the molecular structure of OXD-7. | ||
![Electroluminescence spectra of the [ITO/PYR-7/OXD-7/MgIn] cell (solid line), and the [ITO/PYR-9/OXD-7/MgIn] cell (broken line), respectively.](/image/article/2000/JM/a903239h/a903239h-f5.gif) | ||
| Fig. 5 Electroluminescence spectra of the [ITO/PYR-7/OXD-7/MgIn] cell (solid line), and the [ITO/PYR-9/OXD-7/MgIn] cell (broken line), respectively. | ||
| Compound | PL peak wavelength/nm | Emission color | Maximum luminance/cd m−2 | Efficiency/cd A−1 |
|---|---|---|---|---|
| PYR-7 | 461 | Blue | 1700@15 V | 2.47 |
| PYR-9 | 490 | Blue-green | 5900@12 V | 4.00 |
4 Conclusions
A variety of molecular materials have been synthesized and applied for EL cells. Several kinds of metal complexes were found to be fluorescent and sublimable in vacuum. Particularly, Bebq2, Znq2, 1AZM-Hex and Zn(BTZ)2 showed bright emission in the conventional EL structure [transparent anode/hole-transport layer/emitting layer/cathode]. The maximum luminance of the EL cell in which Bebq2 was used for the emitting layer was 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2, which is bright enough for practical use. The EL cell in which 1AZM-Hex was used showed pure blue emission of 1460 cd m−2. The strong luminescence of these complexes originated in the conjugated systems of the ligand molecules. On the other hand, Eu(TTA)3(phen) did not emit light effectively in the same type of EL cell, although it has intense red PL. The PL system of this europium complex is different from others, the fluorescence originating from a europium(III) ion.25 This is supposed to be one of the reasons of lower EL efficiency. Nevertheless, a variety of metal complexes showed strong fluorescence and good processability in vacuum vapor deposition. Therefore, it is considered that fluorescent metal complexes are most promising materials for OLEDs.
000 cd m−2, which is bright enough for practical use. The EL cell in which 1AZM-Hex was used showed pure blue emission of 1460 cd m−2. The strong luminescence of these complexes originated in the conjugated systems of the ligand molecules. On the other hand, Eu(TTA)3(phen) did not emit light effectively in the same type of EL cell, although it has intense red PL. The PL system of this europium complex is different from others, the fluorescence originating from a europium(III) ion.25 This is supposed to be one of the reasons of lower EL efficiency. Nevertheless, a variety of metal complexes showed strong fluorescence and good processability in vacuum vapor deposition. Therefore, it is considered that fluorescent metal complexes are most promising materials for OLEDs.Organic compounds which have dimer structures are also useful, for example a triphenylamine dimer, TPD, and a oxadiazole derivative, OXD-7. The newly synthesized pyrazoline dimers were available in vacuum vapor deposition and form fluorescent uniform films. Normally, hole-transport materials are not used for an emitting layer, but, in this case, a hole-transportive pyrazoline compound emitted light effectively when it was combined with an electron-transport material, OXD-7. Strong fluorescence of the pyrazoline derivatives originates in the conjugated system around the pyrazoline rings. In particular, PYR-7 has strong blue PL and showed bright blue EL of 1700 cd m−2.
To summarize, metal complexes and dimer structures have been investigated for OLEDs. These molecular designs were found to be very effective in order to meet the requirements of EL materials. Several kinds of metal complexes and dimers showed excellent EL characteristics when they were combined with suitable carrier transport layers. In designing a conjugated material for practical optoelectronics use, a metal chelate and an oligomer structure are found to be very useful in obtaining a stable thin film.
References
- J. KidoPhys. World, 1999, March, p. 27. Search PubMed.
- R. H. Friend, R. W. Gymer, A. B. Holmes, J. H. Burroughes, R. N. Marks, C. Taliani, D. D. C. Bradley, D. A. Dos Santos, J. L. Bredas, M. Loegdlund and W. R. Salaneck, Nature (London), 1999, 397, 121 CrossRef CAS.
- W. Helfrich and W. G. Schneider, Phys. Rev. Lett., 1965, 14, 229 CrossRef CAS.
- C. W. Tang and S. A. VanSlyke, Appl. Phys. Lett., 1987, 51, 913 CrossRef CAS.
- C. Adachi, S. Tokito, T. Tsutsui and S. Saito, Jpn. J. Appl. Phys., 1988, 27, L269 CAS.
- Y. Hamada, C. Adachi, T. Tsutsui and S. Saito, Jpn. J. Appl. Phys., 1992, 31, 1812 CAS.
- Y. Hamada, T. Sano, M. Fujita, T. Fujii, Y. Nishio and K. Shibata, Jpn. J. Appl. Phys., 1993, 32, L511 CAS.
- Y. Hamada, T. Sano, M. Fujita, T. Fujii, Y. Nishio and K. Shibata, Jpn. J. Appl. Phys., 1993, 32, L514 CAS.
- Y. Hamada, T. Sano, M. Fujita, T. Fujii, Y. Nishio and K. Shibata, Chem Lett., 1993, 905 CAS.
- C. Hosokawa, H. Higashi, H. Nakamura and T. Kusumoto, Appl. Phys. Lett., 1995, 67, 3853 CrossRef CAS.
- C. W. Tang, S. A. VanSlyke and C. H. Chen, J. Appl. Phys., 1989, 65, 3610 CrossRef CAS.
- T. WakimotoS. KawamiK. NagayamaY. YonemotoR. MurayamaJ. FunakiH. SatoH. NakadaK. ImaiTechnical Digest; International Symposium on Inorganic and Organic Electroluminescence 1994 Hamamatsu, 1994, p. 77. Search PubMed.
- Y. Hamada, T. Sano, K. Shibata and K. Kuroki, Jpn. J. Appl. Phys., 1995, 34, L824 CrossRef CAS.
- T. SanoY. HamadaK. ShibataInorganic and organic electroluminescence/EL 96Berlin (preprint for International Conference), 1996, Wissenschaft und Technik Verlag, ISBN 3-928943-98-7, p. 249. Search PubMed.
- J. Kido and Y. Lizumi, Appl. Phys. Lett., 1998, 73, 2721 CrossRef CAS.
- Y. Shirota, Y. Kuwabara, H. Inaba, T. Wakimoto, H. Nakada, Y. Yonemoto, S. Kawami and K. Imai, Appl. Phys. Lett., 1994, 65, 807 CrossRef CAS.
- T. Sano, H. Fujii, Y. Nishio, Y. Hamada, H. Takahashi and K. Shibata, Synth. Metals, 1997, 91, 27 CrossRef CAS.
- T. Sano, Y. Hamada and K. Shibata, IEEE J. Sel. Top. Quantum Electron., 1998, 4, 34 CrossRef CAS.
- J. H. Burroughes, D. D. C. Bradley, A. R. Brown, R. N. Marks, K. Mackay, R. H. Friend, P. L. Burns and A. B. Holmes, Nature (London), 1990, 347, 539 CrossRef CAS.
- A. Kraft, A. C. Grimsdale and A. B. Holmes, Angew. Chem., Int. Ed. Engl., 1998, 37, 402 CrossRef.
- T. Sano, M. Fujita, T. Fujii, Y. Hamada, K. Shibata and K. Kuroki, Jpn. J. Appl. Phys., 1995, 34, 1883 CrossRef CAS.
- Y. Hamada, T. Sano, H. Fujii, Y. Nishio, H. Takahashi and K. Shibata, Jpn. J. Appl. Phys., 1996, 35, L1339 CrossRef CAS.
- T. Sano, T. Fujii, Y. Nishio, Y. Hamada, K. Shibata and K. Kuroki, Jpn. J. Appl. Phys., 1995, 34, 3124 CrossRef CAS.
- C. Adachi, T. Tsutsui and S. Saito, Appl. Phys. Lett., 1989, 55, 1489 CrossRef CAS.
- J. Kido, K. Nagai, Y. Okamoto and T. Skotheim, Chem. Lett., 1991, 1267 CAS.
Footnotes |
| † Basis of a presentation given at Materials Chemistry Discussion No. 2, 13–15 September 1999, University of Nottingham, UK. |
| ‡ Present address: Melville Laboratory for Polymer Synthesis, Department of Chemistry, University of Cambridge, Lensfield Road Cambridge UK CB2 1EW. |
| This journal is © The Royal Society of Chemistry 2000 |