Preservation of main-chain conjugation through BODIPY-containing alternating polymers from electronic interactions with side-chain substituents by cardo boron structures†
Abstract
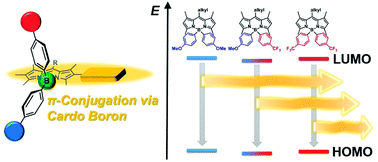
This manuscript describes the synthesis and electronic structures of the modified boron dipyrromethene derivatives containing cardo boron. By using organometallic reagents, the replacement of fluorine groups to aryl substituents with electron-donating groups and/or electron-withdrawing groups was accomplished, resulting in the formation of cardo boron. From the theoretical calculations and optical measurements, electronic structures in the main-chain conjugation were evaluated. In summary, it was clearly shown that cardo boron can efficiently isolate the main-chain conjugation from the electronic interaction with the side chains since the optical properties were highly preserved from the introduction of the side-chain substituents. Our findings should be fundamentally significant for the application of cardo boron-containing conjugated polymers as a scaffold to construct a multi-functional unit by the assembly of functional units.

 Please wait while we load your content...
Please wait while we load your content...